.
Protecting vulnerable persons in research
Necessary protections have two components: fair subject selection, and the specific care required to minimize wrongs to vulnerable persons once they are enrolled in research. Recruitment of research subjects should respect fairness in the distribution of research-related risks and benefits. It is not justifiable to conduct research on an “easily available” population – for example, the rural poor in developing countries – simply because the study will be easier to conduct with them than with persons living in better circumstances. Neither is it defensible to reserve access to potentially beneficial research to the socially privileged. The rationale for the planned recruitment strategy must be based on the balance of potential harms and benefits and the need to obtain generalisable results, and discussed in the protocol.
Specific care requires that the risks and needs of those more likely to suffer wrongs in the conduct of research be identified, and targeted special protections outlined. Protecting vulnerability in clinical research starts with a good grasp of criteria for ethical research, in general. Investigators designing research with vulnerable subjects, and ERCs reviewing such research, should ask themselves the following questions:
(1) In which ways are potential research subjects at risk of being wronged in this research?
(2) Are some potential subjects identifiably more likely than other persons to incur this wrong, or likely to incur it to a greater degree?
(3) Am I/are we among those who share in the duty to minimize, or avoid, this wrong?
(4) If yes, what should we do to avoid this wrong or minimize its increased likelihood or degree, or ensure it is compensated in ethically justifiable ways?
Based on the sorts of wrongs that can occur in clinical research, examples of vulnerability are outlined in Table 31.2; examples of protections tailored to the wrongs involved are outlined in the Table 31.3. In some cases, excluding vulnerable persons from participation in a research project will be an appropriate way to minimize the risk of harm or other wrongs. Sometimes, however, it won’t be. A study designed to address health problems specific to a vulnerable population, such as research on advanced dementia, could benefit the same population of vulnerable persons from which subjects are recruited, and cannot be conducted on nonvulnerable subjects: a condition known as the subsidiarity principle. A study that, although it could be conducted enrolling only nonvulnerable subjects, could be designed in a way to give sufficient extra protection for vulnerable subjects. When such studies offer prospective benefit to research subjects, excluding vulnerable populations rather than providing protections to allow their recruitment can itself be harmful. If a subject in a research protocol with prospective benefit is imprisoned during the study, for example, terminating his participation may not be in his interest. In the rest of this chapter, we focus on two specific populations of individuals often vulnerable in the conduct of research: children and prisoners.
Research involving children
Why are children vulnerable?
Going from the state of being a child to that of being an adult is a continuous process, during which societies identify varying points as the threshold marking the passage from one state to the other. Children are considered vulnerable because, during much of their development, they are incapable of decision-making regarding medical intervention. Even when they are capable of decision-making for clinical care, consent for research requires something more: research-related risks are born for the benefits of others, a fact potential subjects are at risk of misunderstanding even in the best of cases. Although minors who are mature adolescents can often understand the consequences of their choice regarding medical interventions and then provide informed consent for clinical care, this is not considered sufficient in the case of research. Additionally, we are more reluctant to expose children to research-related harms, in part again because the risks undergone in this context cannot be consented to by the child herself, but also because we recognize a general responsibility of protection towards children beyond that which their parents endorse.
What follows from the vulnerability of children for research ethics?
Fair subject selection requires that children be enrolled only when the research question cannot be answered by conducting the study with adults – for example, because the targeted condition is specific to children or because the research question regards the situation of children specifically. When children are recruited in research, protections are required to circumscribe
Table 31.2. Vulnerability as a greater likelihood of being wronged
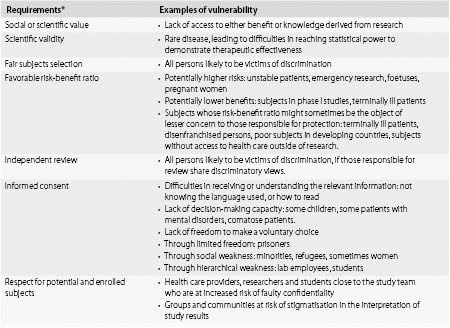
* With permission from Emanuel, E. J., Wendler, D. and Grady, C. (2000). What makes clinical research ethical? JAMA, 283(20), 2701–11.
acceptable risks and to compensate the lack of consent by the children.
Limiting risks to children has implications for the timing of a protocol. Interventions relevant to both children and adults should undergo at least initial testing on adults in order to minimize unknown risks at the time when children will be recruited to assess questions more specific to them. Other implications regard the design of a protocol. Under US regulations, ERCs may approve research involving children under three sets of circumstances: “minimal risk,” “prospect of direct benefit,” and “minor increase over minimal risk.” A prospect of direct benefit is defined as a research situation where risks are justified by the anticipated benefit to the subjects and where the relation of the anticipated benefit to the risk is at least as favourable as that of alternatives available to potential subjects.
Linking the acceptable risk threshold to the prospect of direct benefit in this manner has come under criticism for conflating risks acceptable in therapy and in research, and allowing healthy children to undergo greater research-related risk than those accepted in the case of sick children. An alternative proposed by Wendler is based on the “net risks test.”7 As any assessment of risk in research, it should focus on the research-related risk: risks that potential research subjects would not run outside the protocol. Sick children enrolled in research will often undergo standard therapy as well as experimental interventions, and risks inherent to the standard therapy are not research-related. The assessment should further focus on the net risk: risks that are not balanced by the prospect of direct benefit to the child. Paediatric pharmacokinetic studies, for example, hold no prospect of direct benefit: their entire risk is a net risk. A phase III study of a novel therapy proven effective in adults, however, does hold a prospect of direct benefit for sick children. Such a study can still have a net risk, however, especially if the expected
Table 31.3. Protection of vulnerable subjects by Ethics Review Committees*
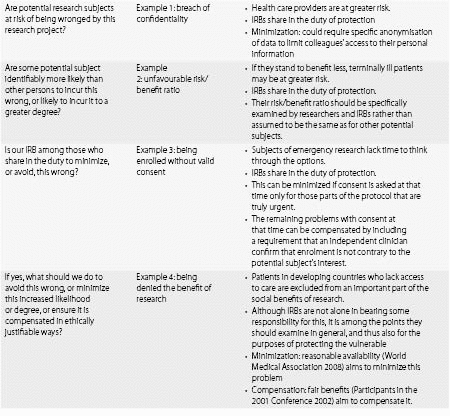
* With permission from Hurst 2008.
benefit is modest. Finally, ERCs should assess this net research-related risk and accept it if it is no greater than “those associated with routine medical and psychological examination”8 or “those ordinarily encountered in daily life.”9 US regulations combine these two thresholds. Comparison of the net research-related risks posed by a study should be with “the level of risk average children face in daily life (or during routine examinations),”10 or the level “normally encountered in the daily lives of people in a stable society”11,12 in order to avoid placing an excessive burden on children suffering from diseases requiring invasive treatment or living in circumstances such as war-torn countries, whose risks in daily life far exceed what is acceptable in research.
Because children are unable to provide consent for their own participation in research, permission must be sought from their parents or other legal guardians. US regulations specify that the permission of both parents is required unless: (1) the investigational procedure involves no more than minimal risk; (2) there is a prospect of direct benefit to the child; or (3) “one parent is deceased, unknown, incompetent, or not reasonably available” or “only one parent has legal responsibility for the care and custody of the child.” EU directive 2001/20/EC specifies that research can only be conducted on minors if “the informed consent of the parents or legal representative has been obtained,”13 apparently requiring the agreement of both parents.
Regulations also require that older children and adolescents should be informed to the level they can understand, and that they should provide assent – defined as “affirmative agreement” in US regulations and provided in writing – when they are capable of doing so.14 Determining when assent should be sought is delicate as children’s ability to understand research participation varies across, but also within, age groups. While it is usually found that children under 7 years of age are not capable of giving assent, and those over 14 years often are, this requires specific assessment, especially in the intermediate age group. Letting families decide when children are old enough to be involved in a decision to enroll in research is one possibility, but it should be applied with caution; data suggest that considerable disagreements can arise within families on this point, including reluctance on the part of the parents of capable children to involve them.15 Children sometimes wish their parents to decide for them, and this, of course, should be respected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




