Regional Anesthesia
Lane C. Crawford
Lisa Warren
I. GENERAL CONSIDERATIONS
A. Peripheral nerve blockade can be an excellent addition or alternative to general anesthesia for many surgical procedures. Regional anesthesia provides optimal surgical conditions of sensory and motor blockade without significantly disrupting autonomic function. Single-shot blocks can provide prolonged postoperative analgesia for up to 24 hours, while continuous catheter techniques can provide analgesia for days. The benefits of regional anesthesia include patient satisfaction, faster initial recovery, and the ability to avoid general anesthesia if desired.
B. The preoperative assessment, preparation of the patient, and degree of monitoring are the same as for general anesthesia. Patients should follow fasting (nothing by mouth) guidelines whenever possible, and regional anesthesia should not be chosen simply to avoid complications related to a full-stomach or difficult airway. Baseline neurologic examination and any preexisting conditions should be documented before any block is performed.
C. Consent for regional anesthesia should include a thorough description of the risks, benefits, options, and common side effects. Need for supplemental local anesthesia, sedation, or potential for general anesthesia should also be discussed.
D. Preoperative anxiolysis may be appropriate as long as the patient remains cooperative and alert. Usually, short-acting agents such as fentanyl and midazolam are adequate. Performing blocks on heavily sedated patients or on adults under general anesthesia is not optimal.
E. Standard ASA monitoring of blood pressure, ECG, and pulse oximetry should be used during performance of all regional blocks. Resuscitation equipment should be readily available, including intralipid for local anesthetic systemic toxicity (LAST) (see Chapter 16).
F. Aseptic technique should be employed during performance of all blocks. Although the definition of essential aseptic technique is robust, evidencebased studies are lacking. Recent reviews and the ASA practice advisory for prevention of infectious complications in neuraxial anesthesia emphasize the following: removal of jewelry and watches, hand hygiene with an alcoholbased antiseptic, use of cap, mask, and sterile gloves, use of individual packets of chlorhexidine solution (preferably alcohol based) for skin preparation, sterile drape of the patient and maintenance of a sterile procedural field, placement of a sterile occlusive dressing over catheter insertion sites, and proper preparation of local anesthetic solutions. The use of a sterile gown has not been shown to reduce bacterial contamination rates compared to the use of gloves alone in several ICU-based studies and remains controversial.
G. Postoperative follow-up should include assessment of block efficacy and duration, patient satisfaction, and presence of residual sensory or motor blockade, paresthesia, and other side effects.
II. GENERAL CONTRAINDICATIONS
Not all patients are suitable for regional anesthesia. Absolute contraindications to regional anesthesia include lack of patient consent, skin infection
at the site of needle insertion, or when nerve blockade would hinder the proposed surgery or desired postoperative neurologic exam. Relative contraindications include coagulopathy, neuropathy, systemic infection, excessive patient anxiety, mental illness, and anatomic distortion. Neuromuscular diseases such as multiple sclerosis, polio, and muscular dystrophy may be aggravated by peripheral nerve blockade.
at the site of needle insertion, or when nerve blockade would hinder the proposed surgery or desired postoperative neurologic exam. Relative contraindications include coagulopathy, neuropathy, systemic infection, excessive patient anxiety, mental illness, and anatomic distortion. Neuromuscular diseases such as multiple sclerosis, polio, and muscular dystrophy may be aggravated by peripheral nerve blockade.
III. COMPLICATIONS COMMON TO ALL NERVE BLOCKS
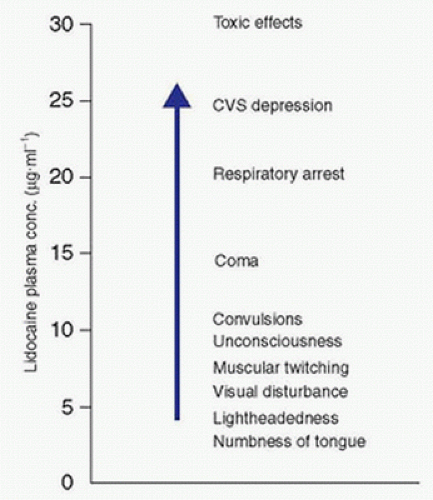
A. Complications of local anesthetics include intravascular injection (Fig. 18.1), local anesthetic systemic toxicity (LAST) (see Chapter 16 Local Anesthetics), and allergic responses. Use of ultrasound, epinephrine in local anesthetic solutions, and intermittent aspiration during injection may help identify intravascular injection. Benzodiazepine premedication increases the seizure threshold and may decrease the central nervous system toxicity of local anesthetics as well as patient anxiety. However, this may also reduce early recognition of toxicity and therefore should be titrated carefully to prevent oversedation.
B. Nerve damage is a rare complication that may result from direct needle trauma, nerve compression and ischemia, or local anesthetic-induced neurotoxicity. Pain with needle advancement or local anesthetic injection suggests an intraneural needle position but is not a reliable predictor. Although pain or paresthesia may also result from external nerve compression by local anesthetic, if encountered during injection, one must consider this complication and redirect needle position.
C. Hematomas may result from vascular puncture but usually resolve without residual problems. When considering regional anesthesia for an anticoagulated or coagulopathic patient, one should consider following neuraxial guidelines, particularly for the deeper blocks (e.g., infraclavicular, lumbar
plexus, paravertebral) where vessel compression for bleeding control is challenging (see Chapter 17).
plexus, paravertebral) where vessel compression for bleeding control is challenging (see Chapter 17).
D. Infection risk is reduced with the use of antiseptic skin preparation and the use of sterile equipment and techniques.
E. Failure/incomplete block can be identified with a careful neurologic examination prior to the beginning of the surgical procedure.
IV. EQUIPMENT
A. Needles Used for Nerve Blockade
1. A block needle should be the smallest diameter possible for patient comfort. However, block needles often are inserted into deep tissue and therefore need a more rigid shaft. For most peripheral regional blocks, a 22-gauge needle is preferable. For superficial blocks, such as axillary blocks, a 23-gauge needle is suitable.
2. Short bevel needles (30 to 45 degrees) are associated with decreased nerve trauma and intravascular injection compared with standard A bevel needles and have become standard for peripheral nerve blocks. However, some data suggest that the use of smaller, sharp needles may be associated with less damage in the event of a nerve injury because of a “clean cut” of the nerve. Newer needles with a Sprotte or Whitacre tip may be less traumatic.
3. Insulated needles designed for use in nerve stimulator-guided techniques have a small conductive area at the needle tip, which allows for more accurate nerve stimulation at lower amplitudes of current compared to noninsulated needles.
4. Echogenic needles designed for use in ultrasound-guided techniques feature modifications such as texturing or scoring of the needle’s surface to enhance reflection of ultrasound waves and improve needle visibility under ultrasound.
5. Ideal needle length varies by block. Upper- and lower-extremity blocks are best performed with a 50- to 150-mm needle depending on the nerve depth. Brachial plexus blocks usually do not require more than a 100-mm needle and can frequently be accomplished, in the case of interscalene block, with a 25- to 50-mm needle.
B. Many blocks require depositing a large volume of local anesthetic with a single injection. Connecting a large-volume (20-mL) syringe to the block needle with sterile extension tubing ensures stable needle position during aspiration and injection. For larger volumes of local anesthesia, multiple syringes can be attached with a stopcock.
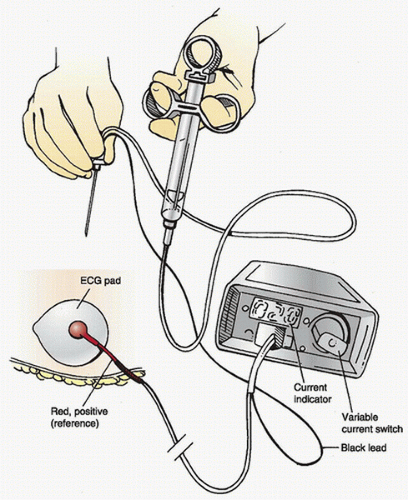
C. Nerve stimulators (Fig. 18.2) designed for regional anesthesia deliver a current of 0.1 to 10.0 mA at a frequency of 1 to 2 Hz and a stimulus duration of 0.1 to 0.3 milliseconds.
D. Ultrasound equipment that is portable and utilizes transducers of various shapes, sizes, and frequencies facilitates imaging of relevant anatomy and serves as an alternative/adjuvant to nerve stimulator approaches. Sterile ultrasound gel and transducer sheaths allow for real-time imaging within a sterile field.
E. A variety of continuous catheter kits and infusion pumps for continuous nerve blockade are commercially available.
F. Selection of local anesthetic should depend upon the desired speed of block onset and duration of block (see Chapter 16 Local Anesthetics).
V. NERVE LOCALIZATION TECHNIQUES
Several techniques may be used to localize target nerves. The classic approach uses anatomical landmarks, tactile feedback from fascial clicks, and elicited paresthesias to guide needle advancement and injection. Nerve
stimulator-guided techniques allow the operator to estimate the distance of the needle tip from the target nerve based on the magnitude of current required to elicit the desired motor response. Ultrasound-guided techniques allow for realtime visualization of the needle tip, relevant anatomy, and local anesthetic spread and have become widely used in recent years. Studies have shown that the use of ultrasound may reduce block performance time, number of needle passes, and volume of local anesthetic required for successful block and may also reduce the incidence of vascular puncture. Nevertheless, complications such as intravascular and intraneural injection have occurred even with the use of ultrasound.
stimulator-guided techniques allow the operator to estimate the distance of the needle tip from the target nerve based on the magnitude of current required to elicit the desired motor response. Ultrasound-guided techniques allow for realtime visualization of the needle tip, relevant anatomy, and local anesthetic spread and have become widely used in recent years. Studies have shown that the use of ultrasound may reduce block performance time, number of needle passes, and volume of local anesthetic required for successful block and may also reduce the incidence of vascular puncture. Nevertheless, complications such as intravascular and intraneural injection have occurred even with the use of ultrasound.
A. Eliciting a paresthesia by contacting a nerve with a needle is a time-honored method of nerve localization. However, this may cause patient discomfort and possibly a higher incidence of postanesthetic dysesthesia or neuropathy. This technique has become less popular as many anesthetists have transitioned practice to either nerve stimulation or ultrasound guidance.
B. Electrical stimulation of a mixed nerve produces a motor response without significant pain.
1. Ground the positive lead of the stimulator to the patient and attach the negative terminal of the stimulator to the needle.
2. Set the nerve stimulator to an initial current of 0.5 to 1.0 mA and move the needle toward the nerve until a motor response in the desired muscle group occurs. If a twitch is uncomfortable, the current should be reduced. Stimulation of the target nerve at a current of 0.2 to 0.5 mA suggests accurate needle placement for local anesthetic administration. Motor response at less than 0.2 mA is suggestive of intraneural placement, and the needle should be withdrawn slightly.
C. Ultrasound guidance may replace or supplement the above techniques for peripheral nerve blockade.
1. Ultrasound uses high-frequency sound waves to visualize structures and tissues in real-time without ionizing radiation. Within the ultrasound transducer, electric current is applied to a piezoelectric crystal array, which vibrates at high frequency to generate sound waves. As these waves travel out through the tissue being imaged, some are reflected back to the transducer. The intensity and delay in return of the reflected sound waves are used to construct a 2D gray-scale image. Denser substances such as bone reflect more sound waves and appear brighter or hyperechoic. Less-dense substances such as air or fluid reflect less and appear darker or hypoechoic.
2. “Knobology”: Commercially available ultrasound machines allow the operator to adjust a number of parameters to optimize visualization. Depth should be set to the minimum that allows visualization of target structures. Focus should be placed just beyond the depth of the target structures. Gain determines the overall brightness of the image and may be increased or decreased as needed. Frequency affects both image resolution and depth of penetration. Higher frequencies increase the resolution at the expense of penetration (suitable for highly detailed examination of superficial tissues such as the breast and thyroid). Conversely, lower frequencies increase the depth of penetration at the expense of resolution (suitable for examination of deep structures such as the heart, abdominal viscera, and uterus). Most nerve blocks (e.g., infraclavicular, femoral, and sciatic nerve at the popliteal fossa) are performed at an intermediate depth and thus utilize intermediate frequencies. Axillary and interscalene blocks are more superficial and are best imaged at higher frequencies. In obese patients, frequencies may need to be decreased to favor penetration, particularly for infraclavicular, neuraxial, and popliteal fossa blocks. Color Doppler is useful for identification of vascular structures.
3. Performance of the block depends on optimal visualization of target structures, proper needle positioning, and adequate spread of local anesthetic around the nerve(s).
a. Initial positioning of the ultrasound transducer is determined by surface landmarks and refined by scanning to obtain the optimal view of the anatomy in question. The left-right orientation of the transducer in relation to the image display should be confirmed prior to scanning. Basic scanning maneuvers include sliding the
transducer in the horizontal plane, tilting its vertical axis to various angles in relation to the skin surface, rotating it around its vertical axis, and applying more or less vertical pressure to it. Color Doppler may be employed to identify vascular structures in the intended needle trajectory. Anatomic structures may be viewed in a sagittal, transverse or an oblique plane to facilitate performance of a directly guided nerve block.
transducer in the horizontal plane, tilting its vertical axis to various angles in relation to the skin surface, rotating it around its vertical axis, and applying more or less vertical pressure to it. Color Doppler may be employed to identify vascular structures in the intended needle trajectory. Anatomic structures may be viewed in a sagittal, transverse or an oblique plane to facilitate performance of a directly guided nerve block.
b. There are two options for needle visualization. In the “out-of-plane” approach, the needle is inserted immediately above or below the midline of the transducer and is then advanced perpendicular to the plane of the ultrasound beam. This provides a cross-sectional view of the needle tip as a hyperechoic dot. Alternatively, in the “in-plane” approach, the needle is inserted several centimeters lateral to the transducer and is then advanced in the plane of the ultrasound beam, allowing continuous visualization of the length of the needle as a hyperechoic line. While the in-plane approach results in the needle traversing a longer distance, it may minimize the likelihood of contacting nerves, blood vessels, pleura, and other vital structures during needle passage.
c. The needle tip should be positioned in close proximity to the target nerve. Spread of local anesthetic should be observed both for evidence of intraneural injection, which may result in nerve injury and should prompt adjustment of needle position, and for adequacy of spread. Circumferential spread of local anesthetic around the target nerve suggests injection in the proper fascial plane. For some blocks, multiple injection sites may be needed to achieve the desired distribution of local anesthetic.
VI. CERVICAL PLEXUS BLOCK FOR REGIONAL ANESTHESIA OF THE NECK
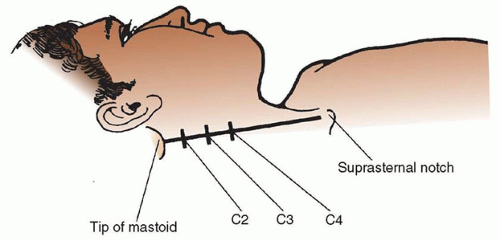
A. Anatomy. The cervical plexus lies in the paravertebral region of the upper four cervical vertebrae (Fig. 18.3). It is formed from the ventral rami of the C1-C4 spinal nerve roots. It is deep to the sternocleidomastoid muscle
(SCM) and anterior to the middle scalene muscle, in continuity with the nerve roots forming the brachial plexus (see section VII.A). The plexus has superficial and deep branches. The superficial branches pierce the cervical fascia anteriorly, just posterior to the SCM, and supply the skin of the back of the head, side of the neck, and anterior and lateral shoulder. The deep branches supply the muscles and deep structures of the neck and form the phrenic nerve.
(SCM) and anterior to the middle scalene muscle, in continuity with the nerve roots forming the brachial plexus (see section VII.A). The plexus has superficial and deep branches. The superficial branches pierce the cervical fascia anteriorly, just posterior to the SCM, and supply the skin of the back of the head, side of the neck, and anterior and lateral shoulder. The deep branches supply the muscles and deep structures of the neck and form the phrenic nerve.
B. Indications. Superficial cervical plexus block produces only cutaneous anesthesia and is useful for superficial procedures on the neck and shoulder. Deep cervical plexus block is a paravertebral block of the C1-C4 nerve roots; anesthetizing both the deep and the superficial branches. Common indications for cervical plexus block are as follows:
1. Cervical lymph node biopsy/excision
2. Carotid endarterectomy
3. Thyroid operations
4. Tracheostomy (when combined with topical airway anesthesia)
C. Complications are possible with deep cervical plexus block because of the close proximity of the needle to neural and vascular structures.
1. Phrenic nerve block is the most common complication. This block should be used cautiously in patients with diminished pulmonary reserve. Bilateral deep cervical plexus block will produce bilateral phrenic and recurrent laryngeal nerve blockade and therefore should be avoided.
2. Subarachnoid injection resulting in total spinal anesthesia.
3. Epidural injection with resultant bilateral cervical epidural anesthesia.
4. Vertebral artery injection causing central nervous system toxicity with very small doses of local anesthetic.
5. Recurrent laryngeal nerve block causing hoarseness and vocal cord dysfunction.
6. Cervical sympathetic nerve block producing an ipsilateral Horner syndrome.
D. Techniques
1. Superficial block with landmark technique: Position the patient supine with the neck slightly extended and the head turned toward the opposite side. Use a 50-mm, 23- to 25-gauge needle to inject 10 mL of local anesthetic subcutaneously along the posterior border of the SCM.
2. Superficial block with ultrasound guidance: Position the patient as above. Place the ultrasound probe transversely over the SCM at the level of the cricoid cartilage and scan posteriorly until the posterior edge of the SCM is visualized in the center of the screen. The supraclavicular nerves may be visualized as two to three small hypoechoic structures between the SCM and scalene muscles. Identify the brachial plexus between the anterior and middle scalene muscles. Insert a 50-mm, 23- to 25-gauge needle just lateral to the probe and advance in plane until the needle tip is beneath the posterior border of the SCM and adjacent or lateral to the plexus. After negative aspiration, inject 10 to 15 mL of local anesthetic and observe spread between the SCM and the underlying prevertebral fascia.
3. Deep block with landmark technique: Position the patient as above. Draw a line connecting the tip of the mastoid process and Chassaignac tubercle (the most prominent of the cervical transverse processes, located at C6, the level of the cricoid cartilage). Draw a second line 1 cm posterior to the first line. The C2 transverse process can be palpated 1- to 2-cm caudad to the mastoid process, with the C3 and C4 processes lying at 1.5-cm intervals along the second line. At each level, insert a 22-gauge 50-mm
needle perpendicular to the skin with caudal angulation. Advance the needle 1.5 to 3.0 cm until it contacts the transverse process. After careful negative aspiration for cerebrospinal fluid (CSF) or blood, inject 3 to 5 mL of local anesthetic solution at each transverse process.
needle perpendicular to the skin with caudal angulation. Advance the needle 1.5 to 3.0 cm until it contacts the transverse process. After careful negative aspiration for cerebrospinal fluid (CSF) or blood, inject 3 to 5 mL of local anesthetic solution at each transverse process.
4. Deep block with ultrasound guidance: Ultrasound may be used to guide performance of the deep cervical block. Although no specific technique has emerged as superior, the goal is to identify the pertinent transverse processes, nerve roots, and boundaries of the cervical paravertebral space and to deposit 3 to 5 mL of local anesthetic adjacent to the nerve roots.
VII. REGIONAL ANESTHESIA OF THE UPPER EXTREMITY
A. Anatomy
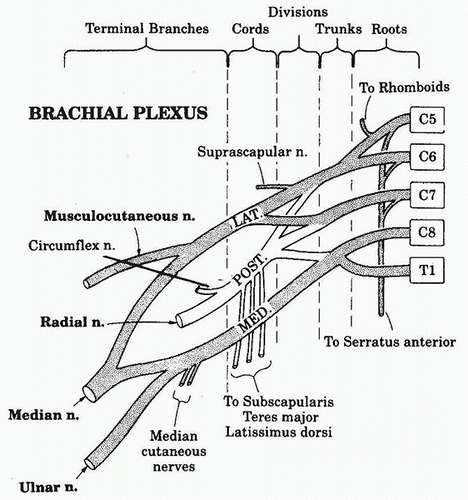
1. The shoulder, axilla, and upper extremity are innervated by the brachial plexus except for the medial aspect of the upper arm that is innervated by the intercostobrachial nerve formed by the second thoracic nerve root.
2. The brachial plexus is formed from the anterior roots of the spinal nerves from C5-C8 and T1, with frequent contributions from C4 and T2.
3. Each root exits posterior to the vertebral artery and travels laterally in the trough of its cervical transverse process, where it is directed toward the first rib and fuses with the other four roots to form the three trunks (upper, middle, and lower) of the plexus. The roots are sandwiched between the fascial sheaths of the anterior and middle scalene muscles.
4. The trunks pass over the first rib through the space between the anterior and middle scalene muscles, in association with the subclavian artery, which shares the same fascial sheath. The roots and trunks have several branches, innervating the neck, shoulder girdle, and chest wall.
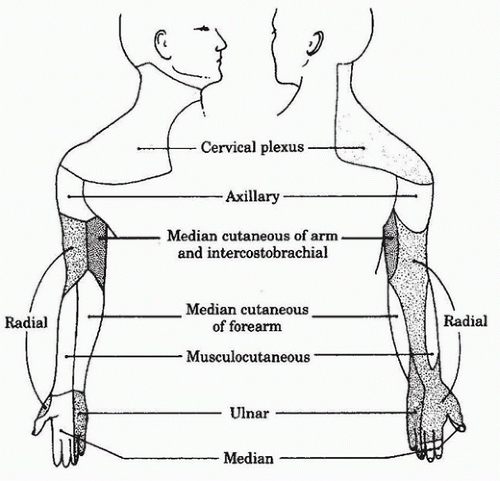
5. As the trunks pass over the first rib and under the clavicle, they split into divisions, which then reorganize to form the three cords ( lateral, medial, and posterior) of the plexus. The cords descend into the axilla, where each has one major branch in addition to several minor branches, before becoming a major terminal nerve of the upper extremity. Branches of the lateral and medial cords form the median nerve. The lateral cord gives off a branch that forms the musculocutaneous nerve, whereas the posterior cord becomes the axillary and radial nerves. The medial cord also forms the ulnar, medial antebrachial, and brachial cutaneous nerves. In the axilla, the median nerve classically lies lateral to the axillary artery, the radial nerve posterior, and the ulnar nerve medial, but variation in these relative positions may occur. The axillary and musculocutaneous nerves exit the sheath high up in the axilla, the musculocutaneous nerve traveling through the substance of the coracobrachialis muscle before becoming subcutaneous below the elbow, and the axillary nerve traveling through the quadrilateral space (bordered by the humeral shaft, long head of the triceps, and teres major and minor muscles) before dividing into its terminal branches. The median cutaneous nerves of the arm and forearm are minor branches of the medial cord (Fig. 18.4). The cutaneous peripheral nerve supply of the upper extremity is summarized in Figure 18.5.
6. The dermatome and sclerotome distribution of the nerves of the body is summarized in Figure 18.6. Cutaneous innervation does not necessarily correlate with deep structures; therefore, knowledge of the sclerotomes can be very useful in predicting the ultimate success of any regional technique.
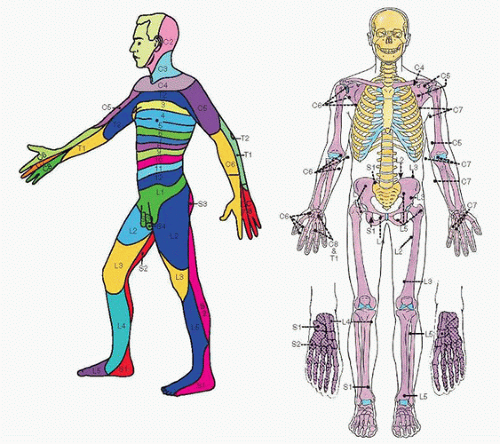 FIGURE 18.6 A side view of the dermatomes (left) and an anterior view of the sclerotomes indicated by the different styles of shading (right). |
7. The major motor functions of the five nerves are as follows:
a. Axillary (circumflex nerve): shoulder abduction (deltoid contraction)
b. Musculocutaneous: elbow flexion (biceps contraction)
c. Radial: elbow (triceps contraction), wrist, and finger extension (extensor carpi radialis longus)
d. Median: wrist and finger flexion (flexor carpi radialis).
e. Ulnar: wrist and finger flexion (flexor carpi ulnaris)
B. Indications
1. Brachial plexus blockade anesthetizes various areas of the upper extremity, depending on which anatomic level of the brachial plexus is blocked. The preferred approach to the plexus depends on the surgical site, the risk of complications, and the experience of the individual anesthetist.
a. The interscalene approach blocks the brachial plexus at the level of the nerve roots to upper trunks. It also blocks the superficial cervical plexus at local anesthetic volumes above 5 to 10 mL, thereby anesthetizing the skin over the shoulder. The ulnar nerve is frequently spared. This approach is most useful for shoulder and proximal humerus surgery. It is less useful for forearm and hand operations, unless supplemented by an ulnar nerve block.
b. The supraclavicular approach blocks the plexus at the level of the trunks to divisions. Because of the compact nature of the plexus at this point of injection and the fact that very few nerves have yet left the plexus, this approach allows for reliable anesthesia of the entire arm distal to the shoulder.
c. The infraclavicular approach blocks the plexus at the level of the cords and provides excellent coverage for surgery distal to the midhumerus.
d. The axillary approach blocks the terminal branches of the brachial plexus. However, because the musculocutaneous and medial cutaneous nerves of the arm exit the sheath more proximally, they are not consistently blocked by this approach, making it unreliable for operations proximal to the elbow.
e. The intercostobrachial nerve, T2, must be blocked in addition to the plexus for procedures involving the medial arm or using a proximal humeral tourniquet. This is accomplished by subcutaneous infiltration with local anesthetic of the medial aspect of the arm at the level of the axillary fossa.
f. Blockade of an individual peripheral nerve may be useful when limited anesthesia is required or a plexus block is incomplete. The musculocutaneous nerve may be blocked at the axilla or the elbow. Each of the other major terminal nerves may be blocked at the elbow, forearm, or the wrist.
C. Techniques and Complications
1. Interscalene
a. Interscalene block using landmark/nerve stimulator technique (Fig. 18.7): Position the patient supine with the head turned slightly away from
the side to be blocked. Identify the lateral border of the sternocleidomastoid by having the patient lift his or her head off the bed. The anterior scalene muscle lies below the posterior edge of the sternocleidomastoid. By rolling one’s fingers posteriorly over the anterior scalene muscle, a groove between the anterior and middle scalenes can be palpated. The intersection of this groove with a transverse plane at the level of the cricoid cartilage is the point at which the needle should enter the skin in a slightly caudad and posterior direction. Because the scalenes are accessory muscles of respiration, asking the patient to take slow deep breaths while palpating for the groove may be helpful. The external jugular vein frequently crosses the groove at the level of the C6 vertebra and may also be a useful landmark. Advance a 25- to 50-mm needle into the groove at a 45-degree angle in a caudal and posterior direction. Stimulation of the plexus will result in a paresthesia or muscle twitch in the deltoid, biceps, triceps, or pectoris major muscles. Paresthesia or twitches confined to the shoulder may result from suprascapular or cervical plexus stimulation and indicate that the needle is posterior to the plexus. Paresthesia or twitches of the diaphragm (hiccoughs) indicate that the needle is just anterior to the plexus. Despite being accurately placed in the groove, the needle will sometimes contact the cervical transverse process without stimulating the plexus. If this happens, withdrawing the needle and redirecting it slightly will likely elicit the correct response. A volume of 30 to 40 mL of anesthetic solution should be injected incrementally after negative aspiration of heme or CSF.
the side to be blocked. Identify the lateral border of the sternocleidomastoid by having the patient lift his or her head off the bed. The anterior scalene muscle lies below the posterior edge of the sternocleidomastoid. By rolling one’s fingers posteriorly over the anterior scalene muscle, a groove between the anterior and middle scalenes can be palpated. The intersection of this groove with a transverse plane at the level of the cricoid cartilage is the point at which the needle should enter the skin in a slightly caudad and posterior direction. Because the scalenes are accessory muscles of respiration, asking the patient to take slow deep breaths while palpating for the groove may be helpful. The external jugular vein frequently crosses the groove at the level of the C6 vertebra and may also be a useful landmark. Advance a 25- to 50-mm needle into the groove at a 45-degree angle in a caudal and posterior direction. Stimulation of the plexus will result in a paresthesia or muscle twitch in the deltoid, biceps, triceps, or pectoris major muscles. Paresthesia or twitches confined to the shoulder may result from suprascapular or cervical plexus stimulation and indicate that the needle is posterior to the plexus. Paresthesia or twitches of the diaphragm (hiccoughs) indicate that the needle is just anterior to the plexus. Despite being accurately placed in the groove, the needle will sometimes contact the cervical transverse process without stimulating the plexus. If this happens, withdrawing the needle and redirecting it slightly will likely elicit the correct response. A volume of 30 to 40 mL of anesthetic solution should be injected incrementally after negative aspiration of heme or CSF.
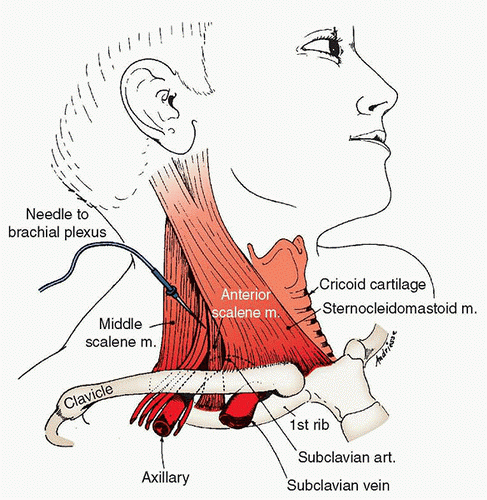 FIGURE 18.7 Interscalene approach to brachial plexus block.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|