CHAPTER 80
Radiofrequency Neurolysis
INTRODUCTION
A careful history and physical examination that implicates the lumbar facets/zygapophyseal joints and its innervations via the lumbar medial branches of the posterior primary rami as the potential pain generators must be clinically established (Figure 80-1).
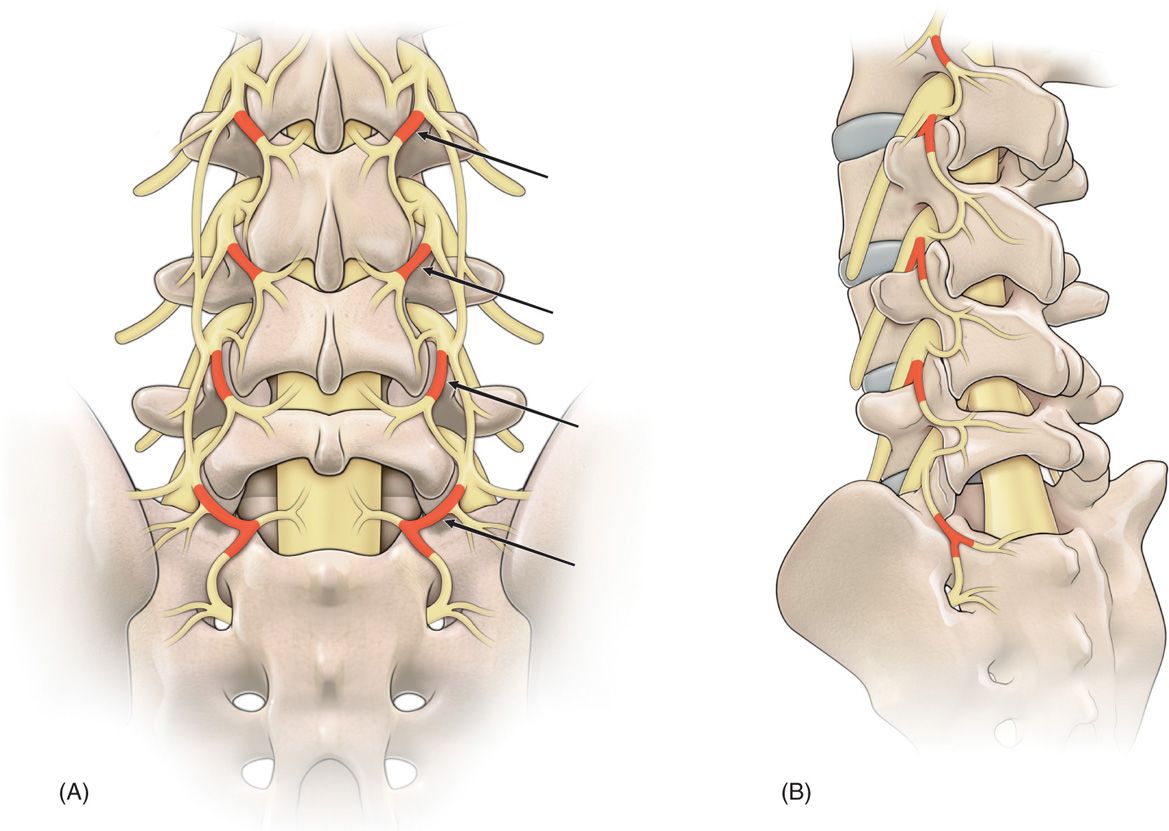
Figure 80-1. Drawings of the nerve supply to the lumbar facet joints (arrows). (A) Anteroposterior view, (B) oblique view.
Physical examination findings that finds direct tenderness on palpation of the skin overlying the lumbar facet joints. Pain is described as deep, dull, achy, diffuse, and in a nondermatomal pattern. Pain may be unilateral of bilateral. Pain is elicited with lumbar spine extension, lumbar spine extension and lateral rotation. Rotational movements, twisting and turning about the axis of the lower lumbar spine causing prevocational pain is suggestive of lumbar facet mediated pain (Figure 80-2).
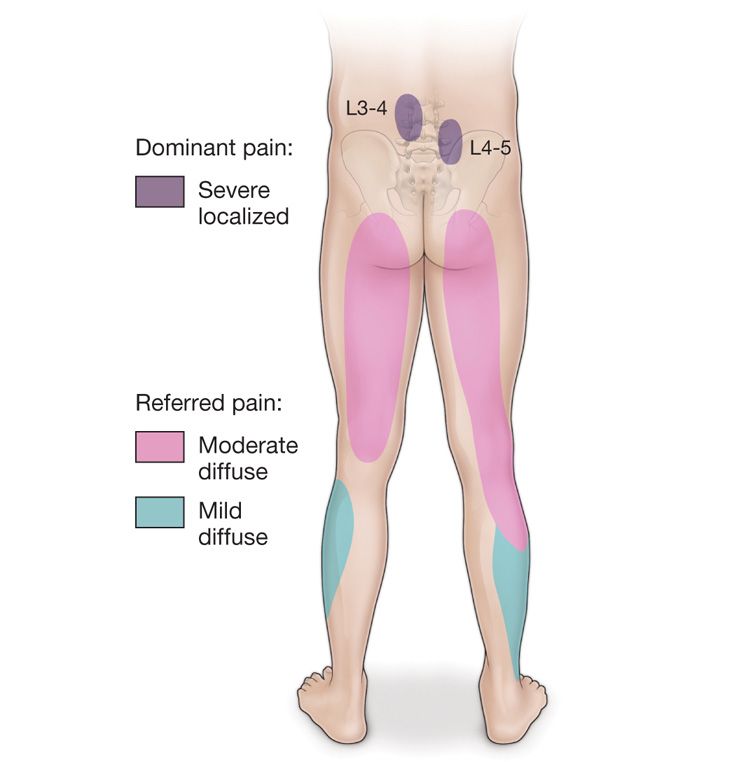
Figure 80-2. Pain distribution in the facet syndrome. Referred pain patterns from facet joints reflect the distribution of the segmental nerve supply at each level involved. Distal reference to the buttocks relates to the caudal migration of posterior branches, whereas limb distribution mimicking root pain results from pain reference in the anterior division of each segmental nerve.
Imaging studies including plain radiographs, computed tomography scanning, and magnetic resonance imaging is used to visualize and demonstrate other potential causes of low back pain. CT scan is the gold standard for visualization of the bony architecture of the facet joint, but it does not imply a specific causative pain generator based on degenerative change, inflammation, synovial cyst or other potential derangement.
INDICATIONS
• When a careful history and physical examination implicate the lumbar facet as a potential pain generator and other pain sensitive structures have been excluded.
• A controlled series of 2 lumbar medial branch blocks is indicated first with a short-acting local anesthetic (1% lidocaine), and then with a long-acting local anesthetic (0.5% bupivacaine).
• There must be a documented benefit from the first short-acting local anesthetic prior to going forward with the long-acting local anesthetic.
• Benefit is described as greater than 70% to 80% positive outcome. This can be measured objectively on a visual analogue or numeric pain scale given to the patient post procedure in the form of a “pain diary.”
• This can also be measured subjectively in terms of improved functionality with physical therapy and basic activities of daily living, range-of-motion, reduction of medication usage, return to work are a few parameters that may be considered when demonstrating benefit.
• If the controlled series of lumbar medial branch blocks are deemed beneficial by the objective and subjective criteria, the next reasonable step in care to provide durable pain relief for the patient is to proceed with radiofrequency neurolysis of the lumbar medial branches of the posterior primary rami of the lumbar facet joints (Figure 80-3).
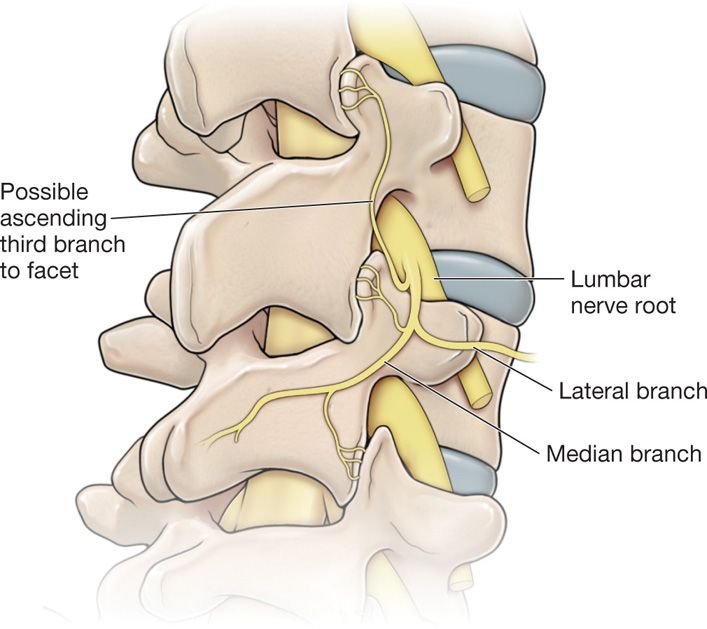
Figure 80-3. This illustration shows that there is a possibility of 3 median nerve branches innervating the one-facet joint. Note the ascending branch from the median nerve supplying the nerve above.
MECHANISM OF NEUROLYSIS
• Radiofrequency neurolysis is the heating of tissue greater than 45°C up to 90°C.
• Temperature generation is achieved via frictional heat that is generated by molecular movement in an electrical field of alternating current above 250 kHz or at radio wave frequency. Hence, the name of the procedure is radiofrequency neurolysis.
• The target tissue around the active tip of an insulated radiofrequency cannula is heated.
• The insulated radiofrequency cannula acts as a heat sink.
• The circuit is completed with a second electrode or the “grounding pad” to allow for current dispersal (Figure 80-4).
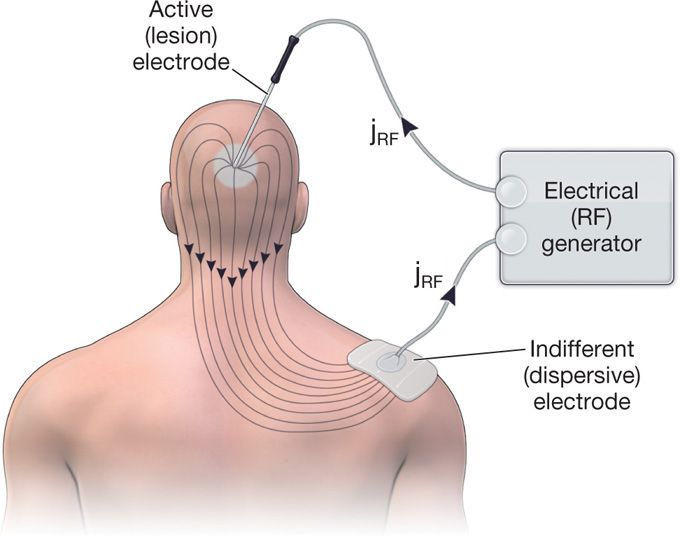
Figure 80-4. The spread of the RF current density (jrf) in the tissue between the active and dispersive electrodes.
CONTRAINDICATIONS
• Patient refusal.
• Localized skin infection over tissue area to be injected and/or systemic infection.
• Coagulopathy (INR >1.4) and/or bleeding diathesis (platelets <50,000/mm3).
• No controlled set (two) of diagnostic medial branch blocks with both a short-acting and long-acting local anesthetic.
• One diagnostic medial branch block is not sufficient to go on to radiofrequency lesioning.
• Allergy to any of the injected components/medication necessary in the diagnostic blocks.
• Pregnancy.
RELEVANT ANATOMY
• The lumbar facet joints/zygapophyseal/z-joints are paired diarthrodial synovial joints located at the posterior border of the spinal column.
• These joints consist of the articulations between the superior and inferior articular processes of the adjacent vertebrae (Figure 80-5).
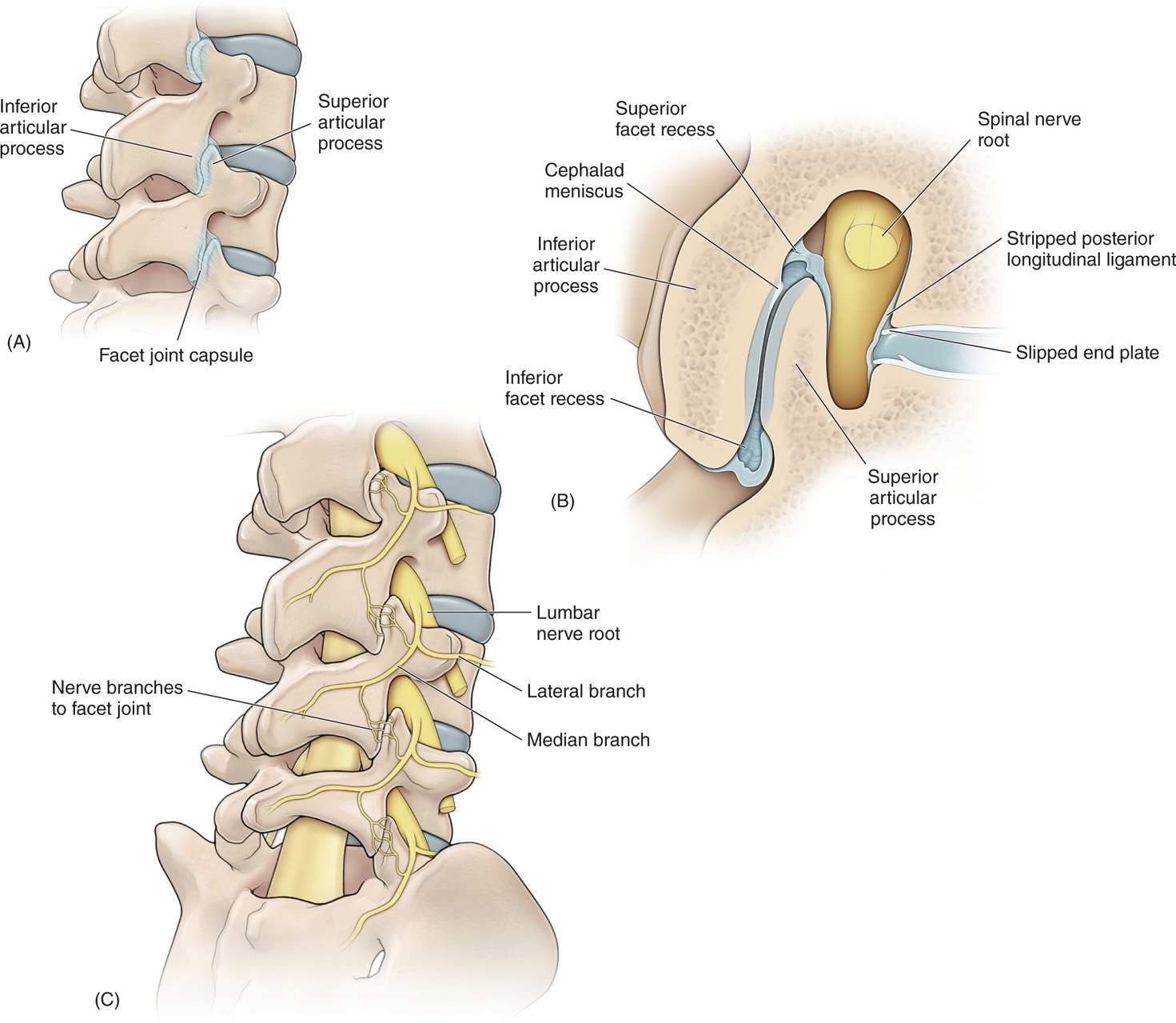
Figure 80-5. (A) Drawing of the lumbar spine in an oblique view identifying the “Scottie dog” in a radiographic image and the relationship of inferior and superior articular processes. Note the facet joint capsule stippling. (B) A cross section of the facet joints shows the details of the facet joint. (C) Drawing of the oblique view of the lumbar spine that shows the lumbar nerve root and the formation of the lateral and median branches of the posterior primary rami. Note the innervation at the facet joint by the median branch.
• The facet joints are true synovial joints having a synovial membrane, joint fluid, and fibrous joint capsule.
• The synovial membrane and joint capsule are richly innervated with sensory fibers including unmyelinated c-fibers (Figure 80-5).
• The specific innervation of each facet joint is by the medial branch of the posterior primary ramus. This small nerve passes over the transverse process and under the mamillo-accessory ligament.
• Each lumbar facet receives innervation from 2 medial branches: one from dorsal ramus above its level and one from its own segmental level (Figure 80-5).
• Hence, when performing either a diagnostic lumbar medial branch blockade or a radiofrequency neurolysis of lumbar medial branches, both dorsal rami must be blocked to achieve denervation of the facet joint and pain relief (Figure 80-5).
PREOPERATIVE CONSIDERATIONS
• American Society of Anesthesiology standard monitoring of the patient: noninvasive blood pressure cuff, ECG, pulse oximetry, and intravenous access.
• Anesthesiologist for administration of conscious sedation or local anesthetic only.
• Fluoroscopic unit C-arm with image storing/printing capability.
• Fluoroscopy or imaging table.
• Radiofrequency generator with simultaneous multilesion capability.
• Radiofrequency insulated cannulae of multiple lengths and gauges: 100, 145 mm (18 and 20 gauge).
• Radiofrequency cannulae 18 or 20 gauge.
• Sterile towels and half-sheet drape.
• Antiseptic skin solution: Betadine or chlorhexidine.
• Preservative-free local anesthetics: 1% lidocaine, 0.5% bupivacaine.
Fluoroscopic Views
• Pillow(s) are used to correct the lumbar lordosis and achieve mild lumbar flexion (Figure 80-6).
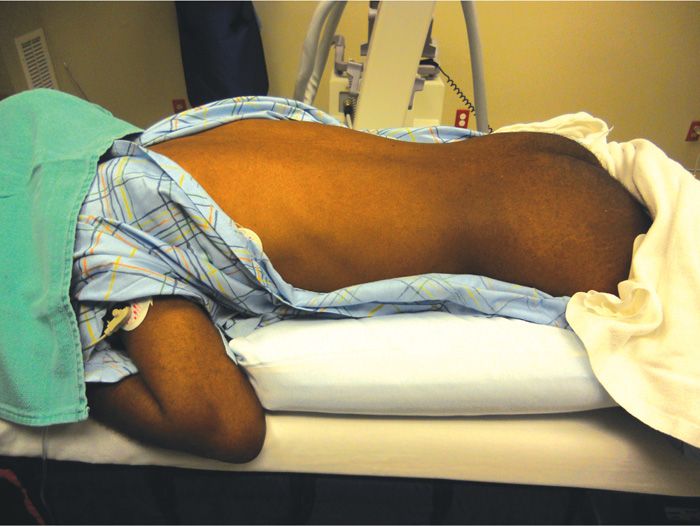
Figure 80-6. Patient positioning correction of lumbar lordosis.
• Anterior-posterior (AP) fluoroscopy views allow visualization of the superior articular process (SAP) and the transverse process (TP) and the pedicle at the level to be blocked or lesioned (Figure 80-7).
Figure 80-7. RF Cannula placement at medial aspect of SAP and TP.
• Use of slight oblique views on the side to be blocked/lesioned (10-25 degrees) allows for better visualization of the SAP and TP.
• Slight caudal angulation (10-25 degrees) after the appropriate oblique view also allows for better SAP and TP visualization.
Patient Positioning
• Prone position with one or more pillows to correct for the lumbar lordosis and create mild lumbar flexion (Figure 80-6).
• Arms placed comfortably in a flexed position toward the head with comfortable padding and support for the elbow joints.
• Head turned to one side whichever is most comfortable.
• Pillow placement distal to the knees if needed for patient comfort.
Selection of Needles, Medication, and Equipment
• Multilesion capable radiofrequency generator (Figure 80-8).

Figure 80-8. RF generator.
• Sterilized RF cables, RF probes of multiple sizes 100, 145 mm (18 or 20 gauge) (Figures 80-9 and 80-10).
Figure 80-9. RF Cannula.
Figure 80-10. RF probe 100 mm.
• Curved tip RF-insulated needles (one for each facet joint to be blocked/lesioned) with a 5- or 10-mm active tip (Figure 80-9).
• Current return or “grounding pad.”
• 1% lidocaine-preservative free for skin wheal and localization prior to lesioning.
• 0.5% bupivacaine or ropivacaine-preservative free.
• 80 mg/mL methylprednisolone.
• Generic nerve block tray with multiple size syringes (5,10 mL) and 18-, 25-gauge needles.
• Record sheet for documentation of lesioning parameters: temperature, impedance, and lesion times.
• Pain diary or log to record subjective and objective response after RF neurolysis.
Interoperative Technical Steps
• Place patient in the prone position on the fluoroscopic table with a pillow at the umbilicus to correct for the lumbar lordosis and create mild lumbar flexion.
• The patient is monitored with standard ASA monitors. Intravenous access is established. Anesthesia is administered and supervised by an anesthesiologist or a supervised nurse anesthetist.
• The patient is prepped and draped in standard sterile fashion and technique with Betadine times 3 or chlorhexidine times 1 in a wide field. Standard towel draping is applied to encompass the operative field. A half-sheet surgical drape is placed from the buttock to cover the field to the end of the fluoroscopic table.
• The C-arm fluoroscopic beam is started in the anterior-posterior position. Start the process with a clear midline view of the lumbosacral spine visualizing the most common targets—the lumbar medial branches at L3-L4, L4-L5, and L5-S1 bilaterally. Isolate the appropriate target site right or left side to start.
• The C-arm is moved in the oblique direction (10-25 degrees) of the targeted medial branches right or left. This allows for visualization of the superior articular process (SAP), transverse process (TP), and pedicle at that level to be best visualized (Figure 80-11).
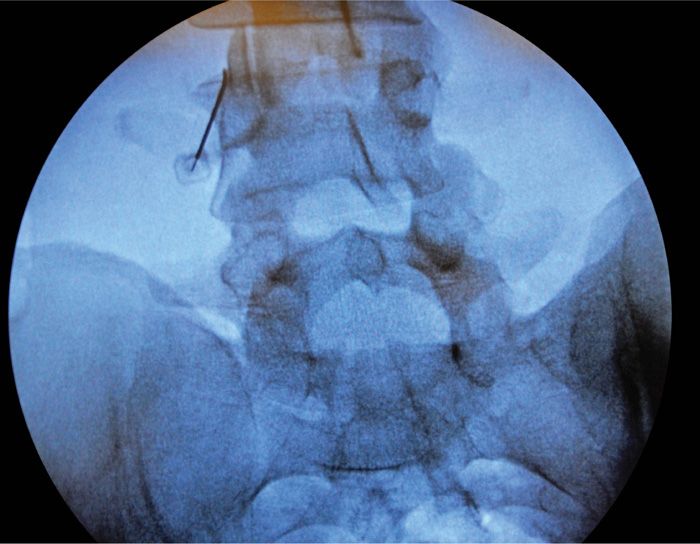
Figure 80-11. Parallel cannula placement for lesioning.
• Caudal angulation (10-20 degrees) of the C-arm will allow for better visualization of the TP and SAP for radiofrequency neurolysis procedures.
• Mark the skin entry target area the inferomedial aspect of the TP as it meets the SAP.
• Create a skin wheal subcutaneous block with 1% lidocaine prior to RF needle insertion.
• Dependent on patient size choose the appropriate RF-curved needle size 100 or 150 mm and 18 or 20 gauge.
• Place the curved RF needle with an inferomedial approach and fluoroscopic guidance at the confluence of the midpoint of the SAP and TP. Take care to place the curved RF needle parallel to the SAP to allow for appropriate lesioning. A perpendicular placement will not allow for accurate medial branch lesioning (Figure 80-11).
• After contact with bone at the superior-medial aspect of the TP-SAP confluence the RF probe lies parallel to the medial branches of the primary posterior rami of the lumbar facet coursing underneath the mamillo-accessory ligament (Figure 80-12).
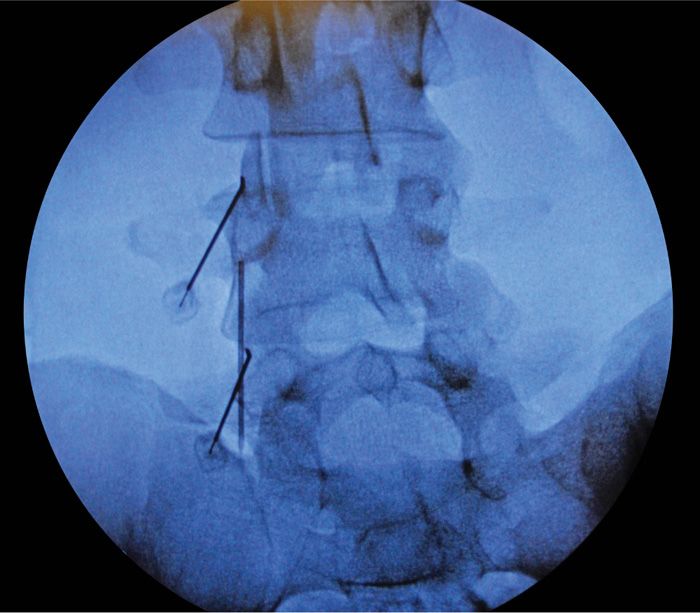
Figure 80-12. Oblique view.
• AP and lateral C-arm views should demonstrate accurate needle position at the medial branch and not near the transforaminal opening.
• Radiofrequency generator is activated and common cable connection is brought to the distal end of the fluoroscopic table (Figure 80-13). Radiofrequency cables are brought from sterile supply and placed on the sterile field and connected to the distal common cable outlet box by an assistant. This way the operator remains sterile (Figure 80-14).

Figure 80-13. RF generator with probe connector.
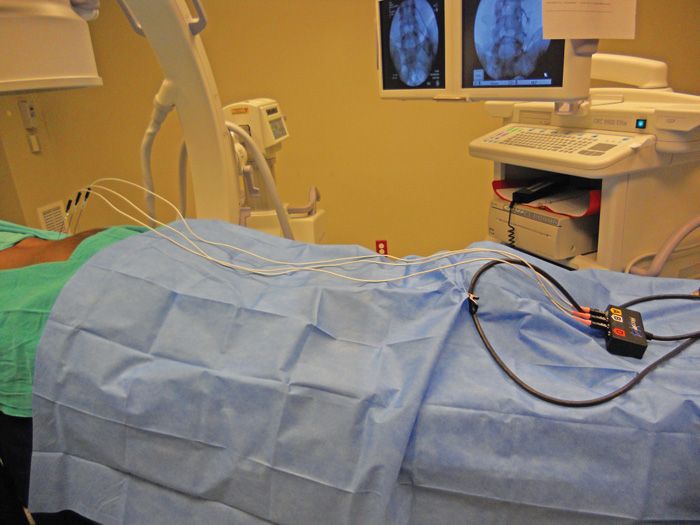
Figure 80-14. Patient positioning with appropriate draping for lesioning.
• Sensory stimulation is carried out at 50 Hz and up to 1.0 mV to reproduce pain in a similar diffuse distribution as typical lumbar facet medial branch mediated pain. There should be no stimulation of the motor or spinal nerves or any radicular pattern of stimulation suggesting proximity to the motor nerve.
• Motor stimulation is carried out at 2 Hz and up to 2.5 V. No motor stimulation or contraction of muscles in the distal extremity should occur nor any radicular stimulation. Localized multifidus muscle stimulation will most likely occur.
• Impedance should be checked and documented. Normal impedance is between 250 and 500 Ω during lesioning. Deep bony contact with RF probes produces high impedances and with blood produces low impedances.
• After confirmation with both sensory and motor testing 1 cc PF 1% lidocaine is placed prior to lesioning to decrease the pain of the lesioning process. (Please note that lidocaine application now eliminates the ability to retest sensory and motor stimulation.)
• Lesioning is carried out at 80°C for 120 seconds to allow for a gentle 30-second ramp up time for the RF probe to reach 80°C. The actual lesioning time is still 90 seconds.
• Direct fluoroscopic visualization is necessary during the lesioning process with spot imagery if there is any change in sensation or untoward stimulation during the RF lesioning process. Needle position can be checked instantaneously.
• The “medial branch” at the L5 position requires special attention. The target is actually the L5 dorsal ramus (there is no L5 medial branch nerve), which is located at the juxtaposition of the sacral ala superior articulating process of S1. The needle is then advanced slightly over the bone into the groove of the sacral ala to contact the dorsal ramus of L5 (Figures 80-15 and 80-16).
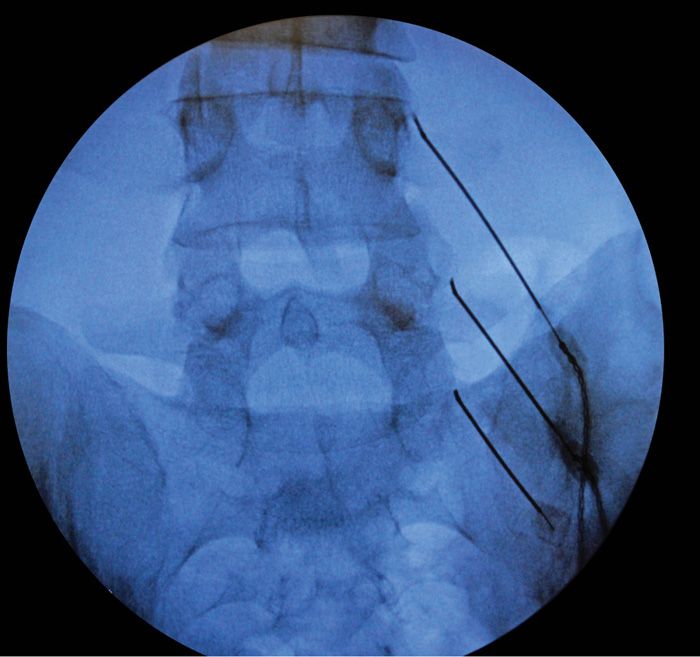
Figure 80-15. Appropriate needle positioning for lesioning.
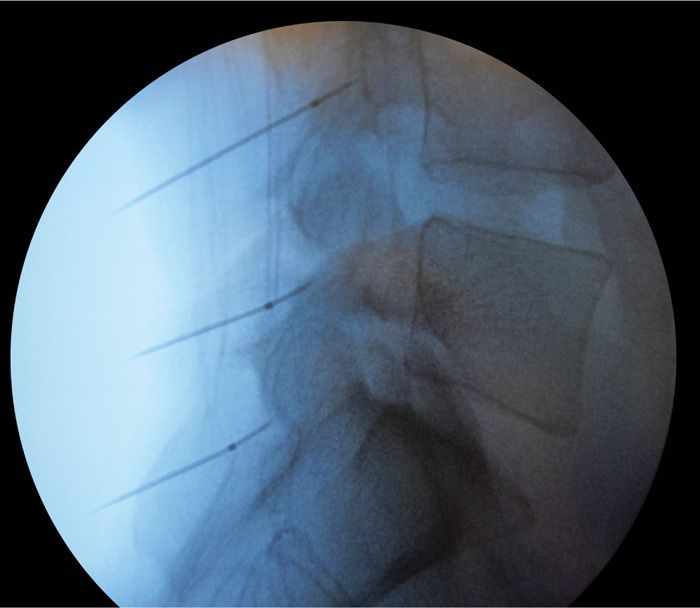
Figure 80-16. Lateral needle placement for lesioning.
• Sensory and motor testing is carried out as specified earlier and RF lesioning is carried out in the same fashion.
• Postlesioning 1 to 2 cc of a solution of admixture of 80 mg methylprednisolone/1 cc and 0.5% preservative-free bupivacaine/1.0 cc at each distinct lesioned site to help limit postprocedure pain.
• Dressings and/or bandages applied to injection sites.
POSTPROCEDURE CONSIDERATIONS
• Standard PACU evaluation, monitoring, and care of the patient postprocedure.
• Examination of the patient in a sitting position regarding pain and response to treatment. Explain and instruct patient on “pain diary” usage postprocedure.
• Examine and treat any postprocedure pain or spasm.
• Give postprocedure pain medications as indicated.
• Verify patient’s ability to ambulate and basic neurological functions are intact.
• Give patient emergency contact information and follow-up visit schedule and time.
• Discharge patient per PACU protocol with a responsible driver.
• No strenuous activity 24 to 48 hours postprocedure.
• Remove bandages in 24 hours. Keep clean and dry until removal.
MONITORING OF POTENTIAL COMPLICATIONS
• Pain, backache, or back spasm. Prescribe short-acting-opioids (SAO) and muscle relaxants (MR) as needed. This is most likely attributed to RF needle insertion.
• Infection. Prescribe short course antibiotics, that patient is not allergic to.
• Muscle bruising or inflammation. Prescribe ice packs and/or NSAIDs.
• Localized bleeding. Change dressing, point pressure usually self-limited.
• Neuritis. None, usually it is self-limited but do a careful follow-up.
• Postdural puncture headache. Provide conservative care, short-acting opioids, NSAIDs and/or muscle relaxants. Perform epidural blood patch if conservative care ineffective.
CLINICAL PEARLS AND PITFALLS
In the carefully selected patient who has undergone a successful controlled lumbar medial branch trial with a greater than 70% to 80% relief, radiofrequency neurolysis provide a durable form of pain relief.
If a patient is a bilateral RF neurolysis candidate and is markedly obese with a difficult airway, it may be beneficial to perform RF neurolysis unilaterally then repeat on the contralateral side in an appropriate time interval for both patient comfort and safety.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






