Chapter 7 Radiofrequency and Other Heat Applications for the Treatment of Discogenic Pain
 Strict selection criteria improve results of annuloplasty procedures for lower back discogenic pain.
Strict selection criteria improve results of annuloplasty procedures for lower back discogenic pain. Patients with evidence of one or two levels of disc degeneration on magnetic resonance imaging (MRI) and one or two levels positive on provocation discography are desired candidates for annuloplasty.
Patients with evidence of one or two levels of disc degeneration on magnetic resonance imaging (MRI) and one or two levels positive on provocation discography are desired candidates for annuloplasty. Disc biacuplasty and IDET are both effective minimally invasive annuloplasty procedures. Biacuplasty may provide a procedural advantage over IDET in regard to the degree of disc disruption and changes to disc architecture. It is also easier to place the electrode in biacuplasty.
Disc biacuplasty and IDET are both effective minimally invasive annuloplasty procedures. Biacuplasty may provide a procedural advantage over IDET in regard to the degree of disc disruption and changes to disc architecture. It is also easier to place the electrode in biacuplasty. Initially the biacuplasty introducer is positioned from the oblique view by rotating the fluoroscopy C-arm to position where the superior articular process of the vertebral level below is somewhere between one third and one half the width of the vertebral column. This view allows optimal placement of two electrodes to form a bipolar configuration with approximate distances of 2.5 to 3 cm or less.
Initially the biacuplasty introducer is positioned from the oblique view by rotating the fluoroscopy C-arm to position where the superior articular process of the vertebral level below is somewhere between one third and one half the width of the vertebral column. This view allows optimal placement of two electrodes to form a bipolar configuration with approximate distances of 2.5 to 3 cm or less. Postprocedurally an optimal rehabilitation step-by-step program is required to ascertain a good outcome.
Postprocedurally an optimal rehabilitation step-by-step program is required to ascertain a good outcome. Patients with increased body mass index, a smoking habit, and multilevel degenerative disc disease have less chance to improve long term.
Patients with increased body mass index, a smoking habit, and multilevel degenerative disc disease have less chance to improve long term.Introduction
There are multiple potential sources of lower back pain. In approximately 45% of the cases, such pain appears to be discogenic in origin.1,2 In clinical practice, often more than one cause can be found simultaneously that might be held responsible for the patients’ pain. The development of various minimally invasive treatments for discogenic pain prompts the development of new diagnostic procedures producing better specificity and sensitivity to confirm or refute clinical suspicion that the patient’s pain is of discogenic origin.
Establishing Diagnosis
The diagnosis of discogenic pain frequently remains unclear secondary to its nonspecific clinical features, including persistent, nociceptive low back and groin and/or leg pain that worsens with axial loading and improves with recumbency. These signs and symptoms are insufficient to establish the diagnosis of discogenic pain and to begin a comprehensive treatment plan for the patients with such complaints. To provide definite diagnosis many practitioners use provocation, functional, and analgesic lumbar discography (see Chapters 4 and 5) in conjunction with magnetic resonance imaging (MRI). Although MRI images may assist in visualizing pathological disc changes such as high intensity zones and loss of disc height, the results correlate poorly with clinical findings, leaving open the question of the clear source of the back pain. Currently provocation discography is the only method that may link pathological abnormalities recognized on MRI with the patient’s pain. Relatively high false-positive rates of provocation discography reported in clinical studies have led to lack of clarity in its predictive value.3–5
Anatomy and Pathophysiology
The etiology of discogenic pain is not well understood. However, newer evidence has suggested that various pathophysiological changes may be associated with disc degeneration and in some cases discogenic pain.6,7 Delamination or tearing of the posterior annulus and diffuse dehydration and progressive loss of nuclear material with increasing age are associated with disc degeneration. Physical changes observed in the degenerative disc are also closely associated with biochemical and cellular changes. The degenerating disc continues to produce inflammatory cytokines, including tumor necrosis factor-α (TNF-α), nitric oxide, and matrix metalloprotineases (MMPs).8 Frequently nerve growth restricted to the outer third of the annulus in a normal disc extends farther into the degenerated disc along the fissures and is supported by extent of the vasculature.9–13 Immunohistochemical studies have confirmed that these aberrantly ingrown nerves are of nociceptive origin (C- and A-beta fibers) and are responsible for transmitting pain responses.6,12 The role of inflammatory cytokines is theorized to increase the sensitization of these ingrowth nerves. Elimination of the previously described nociceptive fibers may stop transmission of the pain signal. Therefore a potentially effective measure to reduce discogenic pain is to denervate the posterior annulus or neural supply to the disc.14
Mechanism of Therapeutic Efficacy
Thermal annular procedures were developed in an effort to provide a minimally invasive alternative to spinal surgeries. Thermal energy is delivered to the affected disc via a resistive heating coil (intradiscal electrothermal therapy [IDET], Smith and Nephew, London, UK) or a radiofrequency (RF) catheter (biacuplasty (Kimberly Clark, Atlanta, Ga; discTRODE; ValleyLab, Boulder, Col) in an attempt to denervate nociceptive fibers and coagulate collagenous tissues in the annulus. Scientific evidence to support such mechanisms of action in the literature is lacking.15
The rationale of disc thermal treatment for discogenic pain was largely influenced by the shoulder capsule studies, in which modifying and shrinking the same type of collagen is clearly documented.16,17 Because the annulus is comprised of collagen, heating may cause collagen shrinkage, limit the leakage of inflammatory disc materials, and cause denervation of nociceptive fibers. Collagen shrinkage and denaturation require a temperature of at least 60° to 65° C.16,17 However, at least when it comes to IDET, it seems that temperatures generated during the procedure are insufficient to alter the collagen architecture.18 Tissue modulation, including shrinkage, denaturation, and structural changes to collagen fibers in the annulus to increase annular stability, is one hypotheses proposed to explain the mechanism of action for the thermal treatment of discogenic pain.16,17
Perhaps a more likely mechanism of action for intradiscal thermal minimally invasive procedures is denervation of ingrown nociceptors by neuroablation. Temperatures reached in the disc using biacuplasty or IDET are sufficient to produce denervation, which occurs at 42° to 45° C.19,20 Still, lack of any evidence to confirm that neuroablation is the mechanisms of action for discogenic pain relief continues to be stumbling block to further clinical research in that area.
One means of delivering thermal energy to biological tissues is to use alternating current (AC). It is superior to direct current (DC) electricity since it can be delivered with less resistance and more control. A direct relationship exists between increased frequency of AC electricity and conductance through tissue.21–23 The frequency used to create thermal lesions in modern RF generators is in the order of 400 to 600 kHz. This corresponds to the frequency of radio waves. At RFs, the flow of electrons does not interfere with physiological functions such as depolarization of muscle cells or neurons. During application of RF, the alternating flow of electrical current causes ions in the tissue to move back and forth. This alternating movement by the ions causes molecular vibration within the tissue and results in frictional heating.22,23 This effect is called ionic heating and can lead to thermal injury to the cells when tissue temperature reaches 42° C.24 The extent of cellular damage is a function of temperature and duration of heating. As the temperature increases, there is an exponential decrease in time needed to cause cellular destruction.25 Increase in tissue temperature, produced by ionic heating, is a function of current density, or the amount of current-per-unit area. Current density is greatest at the proximity of the electrode and decreases with increasing distance from the electrode. However, by increasing the power output, current density around the electrode is increased; thus the lesion size produced by ionic heating is limited by the current density.
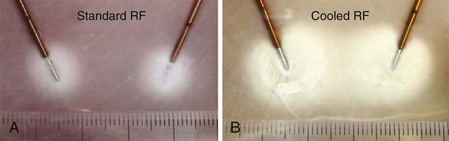
One method of increasing lesion size or volume is by cooling the RF electrode internally (Fig. 7-1). This technique was initially developed for tumor and cardiac ablation26–28 and is currently used in the intradiscal biacuplasty procedure.29–31 Cooled RF probes have hollow lumens that extend to the tip of the electrode. The cooling fluid circulates in a closed loop through the hollow lumens to the tip of the electrode and back to a pump. The coolant acts as a heat sink that removes heat from the tissue adjacent to the electrode. Consequently larger lesion volumes can be produced by increasing power deposition and the duration at which current is delivered without causing tissue charring around the electrode (see Fig. 7-1).26 A larger lesion volume can be produced by using two internally cooled RF electrodes in a bipolar arrangement. Fig. 7-1 compares a bipolar lesion formed with and without cooling. A large confluent lesion can be formed using cooled electrodes at a lower temperature than the small and nonconfluent lesion formed using noncooled electrodes at a higher temperature.
Guidelines
The current body of evidence in peer-reviewed literature does not provide clear support for using intradiscal heat treatments such as IDET and intradiscal biacuplasty for chronic, nonspecific low back pain originating from the intervertebral disc.15 To improve results of minimally invasive intradiscal treatments, it is imperative to use a strict patient selection criteria and provocation discography with manometry appropriately.14 Multiple studies on minimally invasive intradiscal therapies performed in recent years either were pilot trials, or they enrolled patients in a prospective manner but lacked randomization and blinding. This had adverse influence on the interpretation of clinical efficacy by the third-party payers and critics.
Indications
When considering indications for two commonly used annuloplasties, IDET and intradiscal biacuplasty, commonly used patient selection criteria include persistent discogenic lower back pain, which remains despite comprehensive conservative treatment, including physical therapy and a directed exercise program.32–36 Furthermore, no neural compressive lesions should be seen on MRI, and provocation discography should reproduce concordant pain at low pressurization at one or two intervertebral disc levels.33,36 When comparing published IDET studies, differences in outcomes are thought to be related to variability in patient selection.33–36
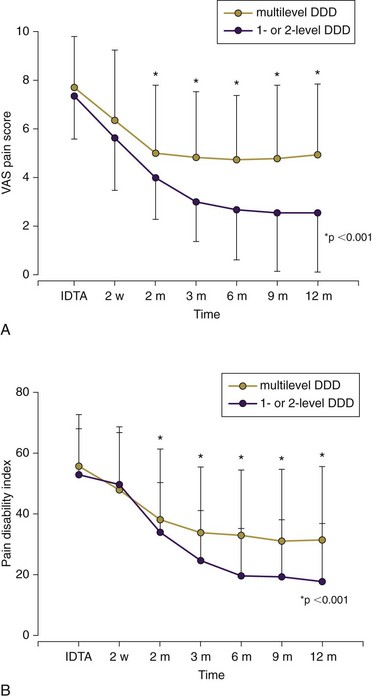
Strict selection criteria should also include Beck’s depression inventory score of <20, less than 20% disc height narrowing on lateral radiograph,37 and any signs of disc degeneration on MRI at more than two lumbar levels.33 Patients with multilevel degenerative disc disease as shown on the MRI study are unlikely to benefit from the annuloplasty (Fig. 7-2). In their randomized, prospective trial Pauza and colleagues37 used provocation discography as a selection criterion rather than a combination of MRI and discography criteria for the enrollment. That may have contributed to somewhat poorer outcomes in this IDET study.37 Overweight patients34 and patients receiving workers’ compensation benefits32,35 represent additional patient subsets that are less likely to improve following any annuloplasty.