Pruritus is mediated via dermal itch receptors, and knowledge of these receptors is rapidly evolving. Until recently believed to be a relatively undifferentiated receptor, it is now recognized that itch receptors are specialized similar to nociceptors. Itch receptors in primates are highly specialized to detect specific itch stimuli and send their input paired with nerve fibers carrying mechanical, thermal, or chemical nociception.[4]
Although the nerve fibers conducting itch sensation initially travel with fibers conveying nociceptive input, they synapse with second order neurons unique for itch in the dorsal horn of the spinal cord.[5] In rodent models, these second order neurons display a gastrin-releasing peptide receptor. The gene for this receptor has been identified, and mice bred without this gene do not display a scratch response to normally itchy stimuli.[6] Targeting this receptor could one day provide efficacious therapy for pruritus in humans. However, contemporary therapy is limited to targeting non-specific transmitters and inflammatory mediators either peripherally at the receptor level or centrally at the level of the spinothalamic tract.
The origin of pruritus may be dermal, neurogenic, psychogenic, neuropathic, or mixed.[7] As with pain, pruritus has the capacity to become neuropathic, causing a chronic pruritus which is difficult to treat. Effective perioperative pruritus therapy should be a clinical priority.
Management: Postoperative pruritus therapy currently utilizes a wide variety of pharmaceuticals with differing mechanisms of action and limited efficacy. No single agent is efficacious for all causes of pruritus or in all patients for a particular cause. Although the symptoms occur at the skin, postoperative pruritus is primarily neurogenic in etiology. Therapy should be directed at the neurogenic cause of pruritus. Topical creams and ointments are suitable for treatment of pruritus of dermal origin, but are generally not effective in the treatment of postoperative pruritus.
Pharmacological pruritus
Opioids: Opioids cause pruritus among 10% to 50% of patients with parenteral administration and between 20% and 100% with neuraxial administration.[8] Severe pruritic symptoms are experienced by only 1% of patients after neuraxial opioid administration, but therapy may be challenging for these patients.[9] Opioid-induced pruritus is neurogenic and occurs without rash or overt skin manifestations.
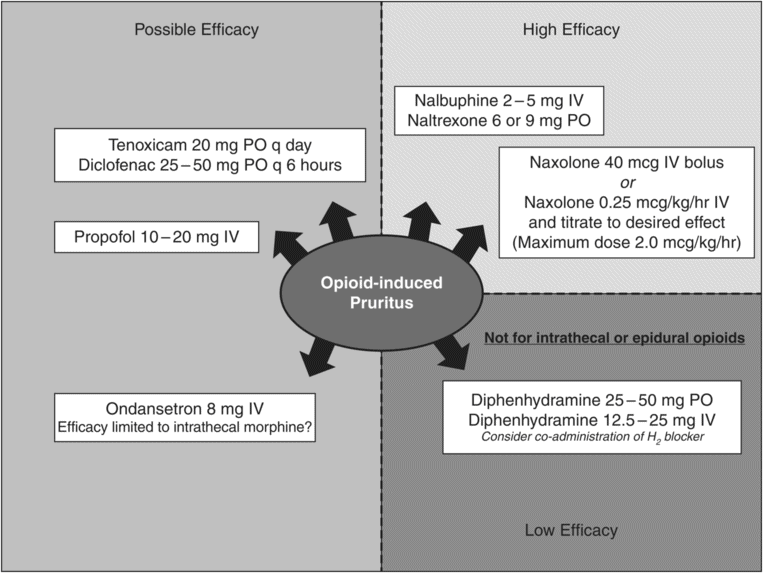
Opioids cause neurogenic pruritus primarily through action on the μ-receptor, although dopamine (D2) receptors, serotonin (5-HT3) receptors, γ-aminobutyric acid (GABA) receptors, glycine receptors, and prostaglandins have been identified as also being involved.[10] Complete μ-receptor antagonism may be achieved clinically with opioid antagonists and will effectively reverse both opioid-induced pruritus and opioid-induced analgesia, which is not tolerable for most surgical patients. Therefore, management of opioid-induced pruritus either targets the μ-receptor with the aim of incomplete antagonism in order to treat pruritus without significant interference of analgesia or aims for blockade of one of the other involved receptors of lesser importance not crucial to analgesia (Figure 20.2). Management is further complicated by the fact that most studies have examined agents for prophylaxis of pruritus; there are too few studies examining the efficacy of rescue therapy for established postoperative pruritus to make evidence-based recommendations.[8]
Incomplete antagonism of the μ-receptor may be achieved either by ultralow-dose administration of an antagonist or by administration of a mixed opioid agonist–antagonist medication. Naloxone 40 mcg intravenous (IV) bolus may be administered for transient relief of opioid-induced pruritus. Continuous infusion of naloxone allows for dose titration and a longer duration of action. Naloxone infusions in the range of 0.17–2.0 mcg/kg/hr have reported efficacy with minimal reversal of analgesia. In most clinical situations, naloxone 0.25 mcg/kg/hr is an appropriate starting dose with slow upwards titration as needed to control symptoms.
Administration of a mixed agonist–antagonist such as nalbuphine or naltrexone may also successfully treat opioid-induced pruritus without reversal of analgesia by incomplete antagonism of the μ-receptor. Competitive binding at μ-receptors may reverse respiratory depression or pruritus (effectively functions as antagonist), but partial agonist effects at kappa-receptors may maintain analgesia. Nalbuphine 2–5 mg IV has been demonstrated to provide effective rescue therapy for pruritus for adults after intrathecal administration of morphine, but efficacy could not be demonstrated in pediatric patients at a dose of 50 mcg/kg IV for treatment of pruritus from intravenous morphine.[10] Naltrexone 6 mg or 9 mg (but not 3 mg) PO is effective as well.[8]
The other receptors identified as involved in opioid-induced pruritus may be targeted, but these receptors appear to be less significant than the μ-receptor and clinically efficacy is also proportionately less. Good clinical results have been achieved by dopamine D2 receptor antagonism. Droperidol 2.5 mg IV reduced the incidence of pruritus from 73% to 40% in one randomized control trial of women receiving morphine 2 mg via epidural catheter after cesarean section.[11] Interestingly, dose escalation to 5 mg IV negatively affects the efficacy of droperidol.[12] IV droperidol might offer some benefit in rescue therapy of pruritus, but the United States Food and Drug Administration’s Black Box warning requires a pre-administration electrocardiogram (ECG) to confirm a normal QT interval followed by 2 to 3 hours of continuous ECG monitoring, which may not be clinically practical in all situations.
Ondansetron 8 mg IV has demonstrated ability to provide prophylaxis for intrathecal morphine, but not intrathecal sulfentanil administration.[5] Non-steroidal anti-inflammatory drugs might theoretically decrease pruritus via an anti-prostaglandin effect. Tenoxicam and diclofenac have some benefit in opioid-induced pruritus, but celecoxib has demonstrated conflicting results in clinical trials.[13] Studies of gabapentin are similarly conflicting with respect to efficacy of pruritus prophylaxis. While gabapentin has some efficacy in the treatment of chronic pruritus, it currently has no role as a rescue medication for pruritus in the perioperative period. Propofol may treat pruritus by inhibition of neuronal transmission at the level of the dorsal horn of the spinal cord. Subhypnotic doses of propofol 10 mg to 20 mg have been evaluated, also yielding conflicting results. Although a single study demonstrated sustained relief of pruritic symptoms (>1 hour) after a propofol 10 mg IV bolus,[14] subsequent studies found either less benefit or no benefit at all.
Although antihistamines have no role for neurogenic pruritus, they may effectively treat dermal pruritus. Morphine, codeine, and meperidine, with oral or parenteral administration, may release histamine and clinically present with a mixed pruritus of dermal and neurogenic origin. H1 antihistamine therapy may benefit the dermal component. H2 antihistamine therapy does not affect pruritus, but does improve the efficacy of H1 antihistamines when administered in combination therapy. Response to antihistaminic therapy is predictably incomplete. Intrathecal or epidural administration of opioids do not release histamine, so this therapy is limited to oral or parenteral administration of certain opioids.[5]
Hetastarch: Hetastarch is a colloid that may be administered perioperatively in order to achieve intravascular volume expansion. Pruritus after administration of hetastarch appears to be common, but ascertaining the actual incidence of hetastarch-induced pruritus is complicated because symptoms may not appear for a month or longer after administration. The clinical association between the hetastarch and the delayed symptomatology may not be recognized in many cases. Hetastarch is most typically administered by anesthesiologists, surgeons, and intensivists, none of whom is likely to be involved in the care of the patient when symptoms develop. It has been estimated that over 12% of patients develop some pruritus after hetastarch administration.[15] The mechanism by which hetastarch causes pruritus is not fully understood, but tissue deposition in or in proximity to small peripheral nerves with direct irritant effect or indirect irritation from mediators released by affected cells appears to contribute.[16] Severe symptoms may be experienced by nearly one-half of affected patients, and symptoms may persist up to 2 years. Despite presentation late in the postoperative period, hetastarch-induced pruritus may occasionally be recognized in the first few postoperative days. Treatment is often ineffective, but there is anecdotal evidence of response to topical capsaicin ointment[17] or naltrexone.[18]
Vancomycin: Rapid administration of IV vancomycin may be accompanied by intense pruritus, cutaneous flush and hypotension (“red man syndrome” – see Chapter 30). Management is to discontinue the infusion and, after symptoms resolve, resume at a slower infusion rate.
Miscellaneous: Any medication causing a Type I hypersensitivity reaction (anaphylaxis) will cause pruritus that is usually associated with urticaria and other constitutional symptoms. Almost all medications may cause anaphylaxis, but the most commonly implicated causes of perioperative anaphylaxis are neuromuscular blocking agents, latex, and antibiotics.[19] Similarly, acute transfusion reaction may present with pruritus and urticaria and other constitutional symptoms. Many medications have some potential to cause pruritus, particularly during the polypharmacy of the perioperative period. Medications commonly initiated perioperatively implicated in pruritus include: angiotensin-converting enzyme inhibitors, angiotensin II antagonists, β-blockers, calcium entry blockers, fractionated heparins, antibiotics, tricyclic antidepressants, carbamazepine, non-steroidal anti-inflammatory drugs, and corticosteroids.[20] Evaluation of new-onset pruritus should include a search for these newly initiated medications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree