Prosthetic Valve Dysfunction
Michael H. Crawford
There are two basic types of prosthetic valves: bioprosthetic and mechanical.
Bioprosthetic valves rarely alter the normal cardiovascular physical examination, and disease of bioprosthetic valves presents with the same physical examination findings as are seen with native valves. Thus, significant regurgitation and stenosis can be detected, but the severity of these lesions can be difficult to assess by physical examination alone.
Mechanical prosthetic valves alter the normal cardiac physical examination. Mechanical prosthetic valve closure sounds are accentuated, and certain types of mechanical prosthetic valves may produce opening sounds that are not normally heard with native valves.
Because prosthetic valves often have smaller orifices than native valves, flow murmurs are frequent and do not necessarily indicate stenosis. Regurgitant murmurs are almost always pathologic, but valvular leaks cannot be differentiated from perivalvular leaks by physical examination.
The baseline physical examination can be confusing in patients with prosthetic valves. The physical examination is most valuable when changes in auscultation of the valve are detected during follow-up. Such changes would suggest valve dysfunction and indicate that echocardiography (echo) is needed.
By the time significant alterations in the cardiac physical examination are detected, often valve dysfunction is marked and the patient is not doing well.
The physical examination is therefore of limited value for early detection of prosthetic valve dysfunction. This makes routine echo an attractive option, but there are little data to support this practice.
Classification of Prosthetic Valves
Bioprosthetic Valves
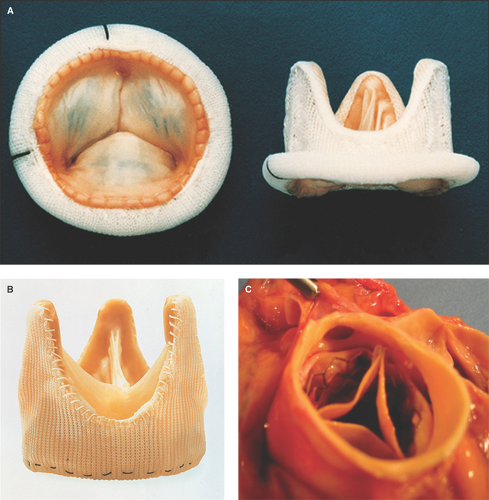
Stented valves are fashioned from animal valves or pericardial tissue and supported by a synthetic framework (Fig. 11.1A).
Stentless valves are created from unsupported animal tissue (1) (Fig. 11.1B).
Stent-mounted bioprosthetic valves are for percutaneous placement, more commonly in the aortic position (Fig. 11.1C).
Homographs are human valves and are autografts (from the same patient) or allografts (from another human).
Mechanical Valves
Bileaflet valves, the most commonly used valves, have two semicircular leaflets attached by hinges to the supporting apparatus and sewing ring (2,3) (Fig. 11.2A).
Single-tilting disc valves are constrained by a series of struts (Fig. 11.2B).
Ball valves are contained by a cage (Fig. 11.2C).
Annuloplasty Rings
Annuloplasty rings are routinely used after mitral and tricuspid valve repair (Fig. 11.2D).
Normal Hemodynamics of Prosthetic Valves
The peak and mean velocities, corresponding gradients, and valve area of normally functioning prosthetic valves vary according to their position, type, and size (Table 11.1).
Prosthetic Valve Dysfunction
Definition
Mechanisms of Prosthetic Valve Dysfunction
Surgical complications: Partial or complete valve dehiscence resulting in paravalvular leak or valve escape.
Mechanical failures: Stent fracture, disc or poppet escape, stuck disc or leaflet (usually in an open position).
Tissue degeneration: Usually results in regurgitation, but stenosis commonly occurs.
Valve regurgitation: Usually results from tissue degeneration, endocarditis, thrombus, or pannus ingrowth.
Valvular stenosis: A less common complication due to tissue degeneration, pannus ingrowth, thrombus, and rarely from endocarditis.
Valve thrombosis: Occurs mainly with mechanical prostheses, usually with subtherapeutic anticoagulation, and may result in regurgitation or obstruction.
Pannus ingrowth: Occurs with mechanical and bioprosthetic valves, more commonly with aortic valves, and results more commonly in obstruction, but regurgitation or stenosis and regurgitation can also occur.
Infective endocarditis: Usually results in regurgitation due to direct leaflet involvement (in bioprosthetic valves) or ring dehiscence (in mechanical and bioprosthetic valves), but large vegetation can rarely obstruct the valve.
PPM: Prosthetic valve area is too small for the patient’s size, which results in relative stenosis.
Echocardiography
Appropriate Indications for Use in Patients with Prosthetic Heart Valves
Transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) is performed in patients after valve replacement, according to different clinical scenarios and American College of Cardiology/American Heart Association/American Society of Echocardiography guidelines (Table 11.2).
Characterization of Mechanisms of Prosthetic Valve Dysfunction
M-Mode Transthoracic or Transesophageal Echocardiography
Best Imaging Planes
TTE: Parasternal long-axis view for aortic and mitral valves; parasternal right ventricle (RV) inflow view for the tricuspid valve; parasternal RV outflow view for the pulmonic valve.
Table 11.1 Normal hemodynamics of prosthetic valves
Prosthesis/Size
Peak Velocity (m/s)
Mean Gradient (mm Hg)
Valve Area (cm2)
Aortic Valve
Mechanical bileaflet
21–23 mm
2.6 ± 0.4
10–30
1.3–1.5
25 mm
2.5 ± 0.4
5–30
1.8
27 mm
2.4 ± 0.4
5–20
2.4
29 mm
2.4 ± 0.4
5–15
2.7–3.6
Stented porcine
21–23 mm
2.6 ± 0.4
13 ± 6
1.8–2.1 ± 0.2
25 mm
2.5 ± 0.4
11 ± 2
—
27 mm
2.4 ± 0.4
10 ± 1
—
29 mm
2.4 ± 0.4
—
—
Stentless porcine
2.2 ± 0.4
3 (2–20)
1.8–2.3
Homograft
1.8 ± 0.4
7 ± 3
1.7–3.1
Mitral Valve*
Mechanical bileaflet
1.6 ± 0.3
5 ± 2
2.9 ± 0.6 (1.8–4.4)
Stented porcine
Hancock
1.5 ± 0.3
4.3 ± 2.1
1.3–2.7
Carpentier Edwards
1.8 ± 0.2
6.5 ± 2.1
1.6–3.5
Homograft
1.8 ± 0.4
6.4 ± 3
1.9–2.9
*Pressure half-time for mechanical and bioprosthetic mitral valves is generally <100 msec and <130 msec, respectively.
Table 11.2 Class I or appropriate (score 7–9) indications for echocardiography in patients with prosthetic valves and suspected prosthetic valve dysfunction
Baseline TTE 1–3 mo after valve replacement.
Routine surveillance at least 3 years after valve replacement if no known or suspected valve dysfunction.
New regurgitant murmur (TTE).
Development of new or changing cardiovascular symptoms (TTE).
Lack of improvement or deterioration of functional capacity or cardiovascular symptoms after valve replacement (TTE).
Yearly after 5 y with a bioprosthetic valve in the absence of a change in clinical status (TTE).
Every 6 mo in asymptomatic patients with bioprosthetic valve degeneration and greater than or equal to mild regurgitation (TTE).
Patients with suspected valve obstruction caused by thrombus or pannus ingrowth (TTE or TEE).
Patients with suspected prosthetic valve endocarditis due to positive blood cultures or new murmur (TTE or TEE).
Re-evaluation of patients with infective endocarditis with any of the following: virulent organism, severe hemodynamic lesion, aorta involvement, persistant bacteremia, a change in clinical status, or symptomatic deterioration (TTE or TEE).
Diagnose or manage endocarditis with a moderate to high pretest probability—for example, bacteremia, especially with staphylococcus or fungi (TEE).
Persistent fever in a patient with an intracardiac device (TEE).
TTE, transthoracic echocardiography; TEE, transesophageal echocardiography.
(Adapted from Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52:e1–e142; Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/HFSA/HRS/ASNC/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166.)
TEE: Basal four-chamber view for mitral valve and tricuspid valve, and basal long-axis views for aortic and pulmonic valves.
Key Diagnostic Features
Abnormal leaflet disc or poppet motion with regard to cardiac cycle; for example, late opening or closing, or failure to close or open.
Thickened prosthetic valve or periprosthetic valve tissue.
Flail bioprosthetic valve leaflet.
Pitfalls
The poor lateral resolution of M-mode echo may superimpose adjacent structures in the image and give the appearance of abnormalities.
Has limited sensitivity and specificity in defining the different mechanisms of prosthetic valve dysfunction.
Two- or Three-dimensional Transthoracic or Transesophageal Echocardiography
Best Imaging Planes
TTE: Parasternal long- and short-axis views and RV inflow and apical views.
2D TEE: Basal four-chamber, long-axis, and short-axis views.
RT3D: Basal four chamber and short axis views for online or offline reconstruction of LA and LV en face views of the mitral valve and aortic root and LV outflow tract en face views of the aortic valve.
Key Diagnostic Features
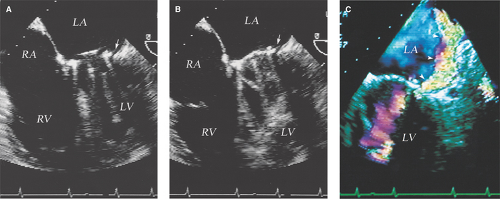
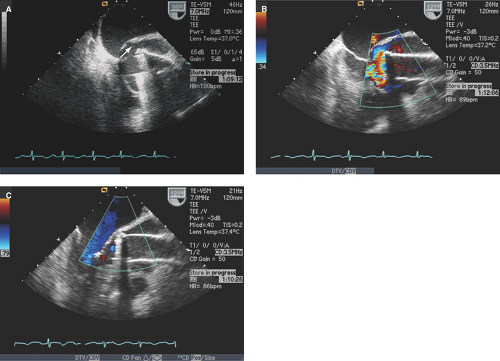
Valve dehiscence: Rocking motion of part or entire prosthetic valve >15 degrees with evidence of separation of the prosthetic ring from the valve annulus (Figs. 11.3 through 11.5).
Bioprosthetic valve degeneration: Thickened or torn/flail leaflets (Fig. 11.6).
Abnormal tissue masses, which include torn leaflets, pannus, thrombi, or vegetations:
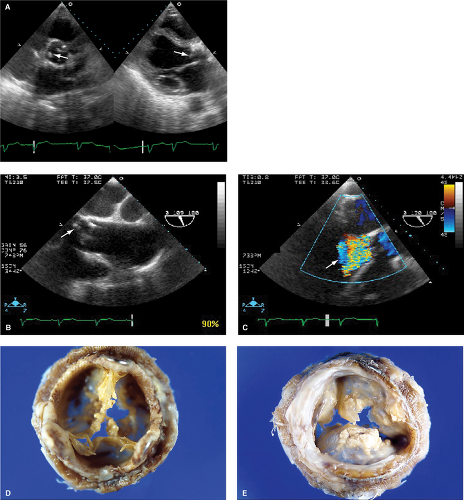
Pannus is generally echo dense with linear or bandlike shape. Pannus usually causes stenosis (Fig. 11.7). Pannus can also cause stenosis with intermittent regurgitation (Fig. 11.8).
Vegetations and thrombus are more commonly masses of soft tissue echoreflectance, of variable shape, and mobile. Both occur more commonly on the sewing or annuloplasty rings (Fig. 11.9). However, infection on the sewing ring can cause ring dehiscence without apparent vegetative masses (4) (Fig. 11.10). Also, diffuse and well-layered thrombosis of bioprosthetic leaflets leading to stenosis can rarely occur (Fig. 11.11).
Abnormal leaflet, poppet, or disc motion.
Analysis of chamber sizes and function.
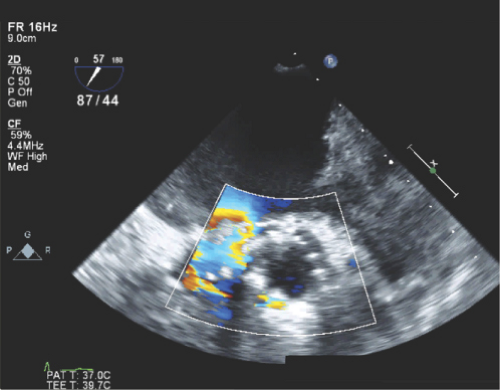
Figure 11.5: TEE basal short-axis view of a St. Jude aortic prosthetic valve with a color flow Doppler signal representing a paravalvular leak in diastole (arrow).