15 Prolotherapy
A CAM Therapy for Chronic Musculoskeletal Pain
Prolotherapy is an injection-based complementary and alternative medicine (CAM) therapy for chronic musculoskeletal pain. The historical roots of therapeutic intervention that may have similar underlying mechanisms of action, however, extend to antiquity. Hippocrates anecdotally described the use of hot wires to create scar tissue (sclerotherapy, an early name for prolotherapy) in cases of severe shoulder dislocation in soldiers. Prolotherapy injections have been used for approximately 100 years by physicians practicing both conventional and complementary medicine. Modern applications can be traced to the 1950s when prolotherapy injection protocols were formalized by George Hackett,1 a general surgeon in the United States based on his clinical experience of more than 30 years. Although techniques and injected solutions vary by condition, clinical severity, and practitioner preferences, a core principle of contemporary prolotherapy is that a relatively small volume of an irritant or sclerosing solution is injected at sites on painful ligament and tendon insertions, and in adjacent joint space over the course of several treatment sessions.1,2 Prolotherapy is becoming increasingly popular in the United States and internationally, and is actively used in clinical practice.3,4 Increased quantity and quality of the scientific literature supporting prolotherapy, internet reports, and the use of prolotherapy by prominent sports personalities all contribute to a growing visibility and interest among physicians and patients. A 1993 survey sent to osteopathic physicians estimated that 95 practitioners in the United States were estimated to have performed prolotherapy on approximately 450,000 patients. However, this survey likely underestimated the true number of practitioners dramatically because only 27% of surveys were returned.5 No formal survey has been done since 1993. The current number of practitioners actively practicing prolotherapy is not known but is likely several thousand in the United States based on attendance at CME conferences and physician listings on relevant websites. Prolotherapy has been assessed as a treatment for a wide variety of painful chronic musculoskeletal conditions that are refractory to “standard of care” therapies. Although anecdotal clinical success guides the use of prolotherapy for many conditions, clinical trial literature supporting evidence-based decision-making for the use of prolotherapy exists only for low back pain, several tendinopathies, and osteoarthritis.
Nomenclature
Nomenclature surrounding prolotherapy has evolved. This procedure, now known most commonly as prolotherapy, has been identified using names consistent with existing hypotheses and understanding of possible mechanisms of action. Nomenclature has reflected practitioners’ perceptions of prolotherapy’s therapeutic effects on tissue. Historically, this injection therapy was called “sclerotherapy” because early solutions were thought to be scar-forming. “Prolotherapy” is currently the most commonly used name, and is based on the presumed “proliferative” effects on chronically injured tissue. It has also been called “regenerative injection therapy” (RIT)2,6; some contemporary authors identify the therapy according to the injected solution.7 The precise mechanism of action of the various injectants is not known, but is likely to be multifactorial.
Professional Status
No single national medical organization acts as a supervisory, credentialing, or guideline-generating capacity for prolotherapy or its practitioners. The National Institutes of Health identifies prolotherapy as a CAM therapy. The NIH National Center for Complementary and Alternative Medicine (NCCAM) has funded two ongoing clinical trials and two completed basic science studies on the mechanisms of prolotherapy.8,9 The Centers for Medicare and Medicaid Services and the Veteran’s Administration have reviewed the prolotherapy literature for low back pain and all musculoskeletal indications, respectively, and determined existing evidence to be inconclusive. Neither agency recommends third party compensation for prolotherapy. However, neither review included the most recent clinically positive studies or reviews in their evaluation.7,10–13 Private insurers are beginning to cover prolotherapy for selected indications and clinical circumstances; however, most patients pay “out-of-pocket”.
Prolotherapy Technique
Like contemporary Western medical therapies, CAM therapies often follow directly from a particular way of understanding the patient’s normal and pathologic conditions. For example, acupuncture relies on an ancient tradition of energy meridians and physiologic correlations that inform the specific procedures of needle placement to improve overall balance. Meditation relies on an understanding of interaction between mind and body to influence the body using mental processes. The same is true of prolotherapy. In his clinical text on the practice of prolotherapy, George Hackett laid out the foundations of treating musculoskeletal injuries using prolotherapy. He developed a diagnostic and treatment strategy familiar to those with a detailed knowledge of conventional Western understanding of the effects of local trauma, healing, and pain referral patterns. His diagnostic and therapeutic techniques, developed in the 1950s, could be viewed as holistic before the term gained popularity in contemporary medicine. Hackett interpretated chronic pain and referred pain symptoms such as numbness, tingling, and weakness, and alteration in proprioception, as originating, in part, from poorly healed ligaments and tendons. As such, he preceded by several decades the current change in the understanding of chronic tendon and ligament pain. Formerly thought to be primarily inflammatory in nature (“tendonitis), chronic tendon injury is now understood to be primarily a result of noninflammatory, degenerative tissue (tendinosis). Injection protocols for a particular body part tend to treat not just the specific injured structure, but also the surrounding structures, in an effort to increase the integrity of the area as a whole.
Clinical Vignette
Patient Description
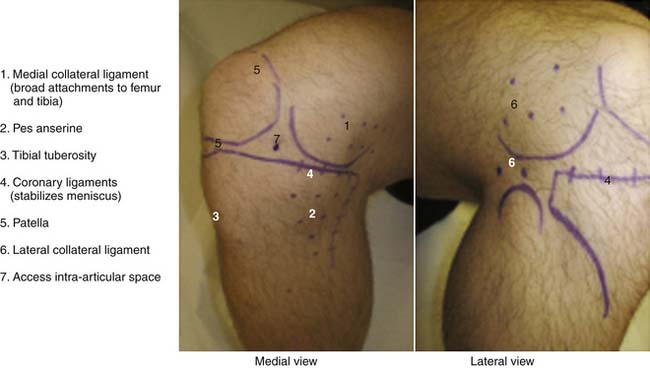
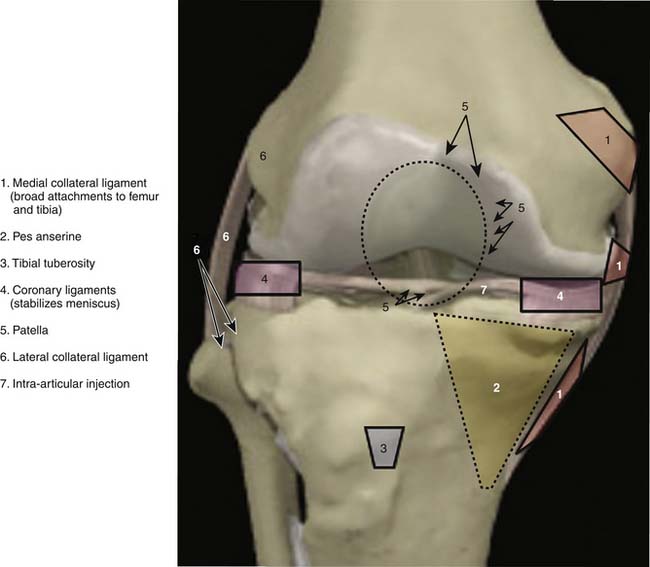
Anatomic marking and local anesthesia: The major superficial anatomic landmarks are palpated and marked with skin marker of choice (Fig. 15-1). Tender areas are injected with small intradermal skin wheals because this makes the deeper injections less painful. One percent buffered lidocaine is often used.
Injection of Ligamentous and Tendinous Attachments
Mechanism of Action
Injected solutions (“proliferants”) have historically been hypothesized to cause local irritation, with subsequent inflammation and tissue healing resulting in enlargement and strengthening of damaged ligamentous, tendinous, and intra-articular structures.14,15 These processes were thought to improve joint stability, biomechanics, function, and ultimately, to decrease pain.1,2
The mechanism of action for prolotherapy is not clearly understood and, until recently, it received little attention. Supported by pilot-level evidence, the three most commonly used prolotherapy solutions have been hypothesized to act via different pathways: hypertonic dextrose by osmotic rupture of local cells, phenol-glycerine-glucose (P2G) by local cellular irritation, and morrhuate sodium by chemotactic attraction of inflammatory mediators16 and sclerosing of pathologic neovascularity associated with tendinopathy.17,18 The potential of prolotherapy to stimulate release of growth factors favoring soft tissue healing has also been suggested as a possible mechanism.19,20
In vitro and animal model data have not fully corroborated these hypotheses. An inflammatory response in a rat knee ligament model has been reported for each solution, although this response was not significantly different from that caused by needle stick alone or saline injections.8 However, animal model data suggest a significant biologic effect of morrhuate sodium and dextrose solutions compared to controls. Rabbit medial collateral ligaments injected with morrhuate sodium were significantly stronger (31%), larger (47%), and thicker (28%), and had a larger collagen fiber diameter (56%) than saline-injected controls14; an increase in cell number, water content, ground substance amount, and a variety of inflammatory cell types were hypothesized to account for these changes.15 Rat patellar tendons injected with morrhuate sodium were able to withstand a mean maximal load of 136% (±28%)—significantly more than the uninjected control tendon.21 Interestingly, in the same study, tendons injected with saline control solution were significantly weaker than uninjected controls.21 Dextrose has been minimally assessed in animal models. Recent studies showed that injured medial collateral rat ligaments injected with 15% dextrose had a significantly larger cross-sectional area compared to both noninjured and injured saline-injected controls.22 P2G solution has received the least research attention; although it is in active clinical use, no animal or in vitro study has assessed P2G effect using an injury model. In research settings, most clinicians report using these solutions as single agents, although concentration of each one varies. In clinical practice, physicians sometimes mix prolotherapy solutions, or use solutions serially in a single injection session depending on experience and local practice patterns. The effect of varied concentrations or mixtures has not been assessed in basic science or clinical studies and no clinical trial has compared various solutions.
Clinical Evidence
Early Research
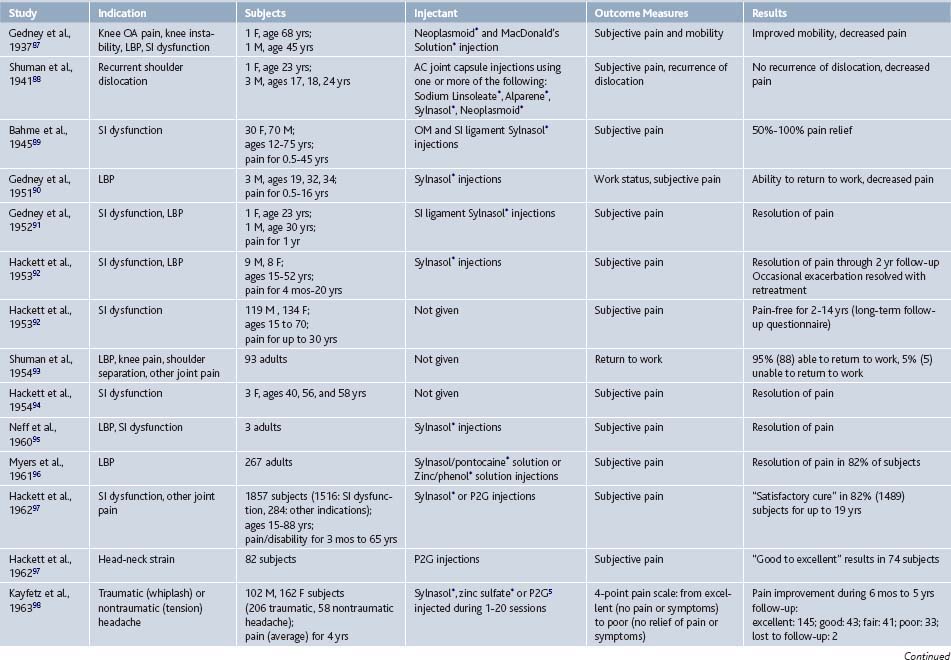
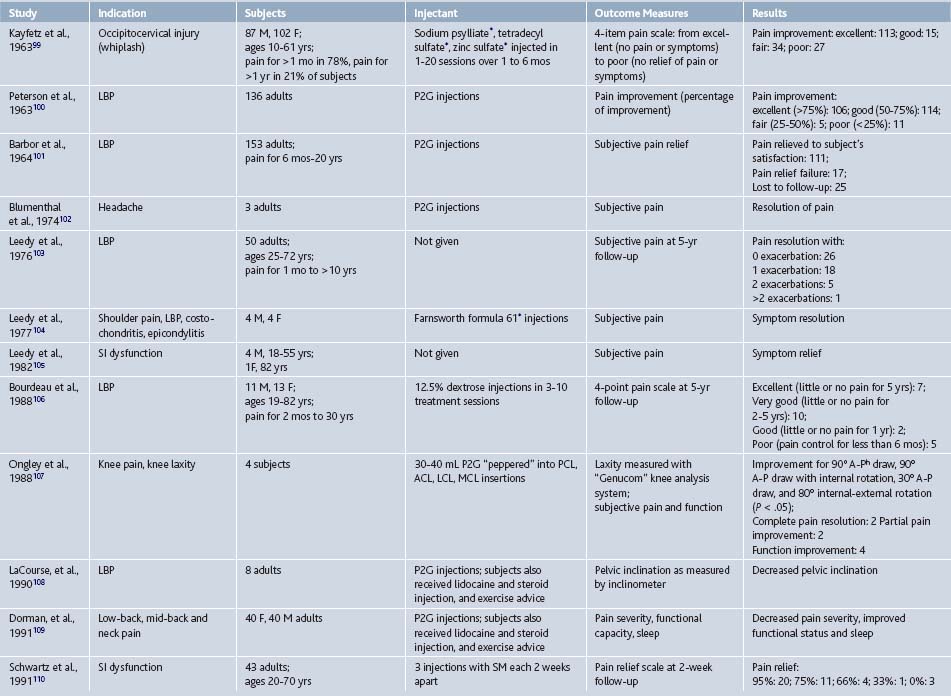
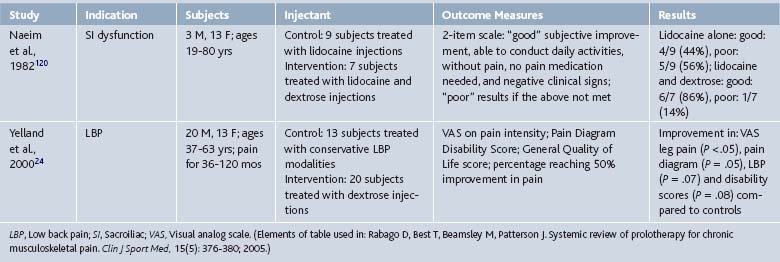
Since its inception, prolotherapy has been primarily performed outside of academia. Research has been primarily done by clinicians who perform prolotherapy themselves. This has lead to a pragmatic orientation of existing prolotherapy studies and a relative paucity of large, rigorous, clinical trials in spite of significant clinical activity. Although the first randomized controlled trial (RCT) did not appear until 1987, clinicians have enthusiastically reported the results of more modest, pilot-level nonrandomized clinical trials since the 1930s (Tables 15-1 and 15-2).
In 2005, our group reported the results of a systematic review of prolotherapy for all indications; we found 42 published reports of clincal prolotherapy trials since 1937.23 Thirty-six of the studies to date were case reports and case series that included 3928 patients aged 12 to 88 years. These uncontrolled studies provide the earliest and most clinically-oriented evidence for prolotherapy. Each study reported positive findings for patients with chronic, painful, refractory conditions. Report quality of the included studies varied widely; their internal methodologic strength was generally consistent with publication date. The older case studies documented several injectants and methods that are no longer in use. Contemporary solutions were noted to start with P2G in the 1960s, dextrose in the 1980s, and morrhuate sodium in the early 1990s. The case reports and case series highlighted the fact that, over time, prolotherapy has been used and studied for a continually growing set of clinical indications. These case studies have also been used as pilots to develop new assessment techniques that could help elucidate pathophysiology of the condition in question7 and test methodology for future, more robust randomized trials.24 In general, while lacking control groups and randomization, these pragmatic studies25 had the advantage of assessing effectiveness of prolotherapy in “real life settings” that patients encounter, including the prolotherapist’s ability to select the patient and to individually tailor the injection protocol. Most of the subjects (72%, 2691 of 3741) assessed in the early literature were treated for low back pain. However, other indications assessed by these early studies included knee osteoarthritis, shoulder dislocation, neck strain, costochondritis, lateral epicondylosis and other tendinopathies, and fibromyalgia (see Tables 15-1 and 15-2).
Contemporary Research
Research on prolotherapy and its clinical effects has accelerated, and the number and methodologic quality of studies assessing prolotherapy have increased dramatically since the mid-1980s (Fig. 15-3).
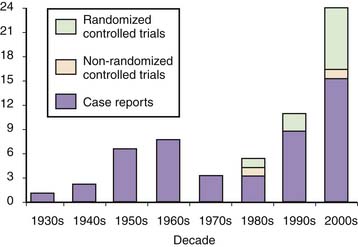
Figure 15-3 Number of published clinical studies on prolotherapy since 1937.
(Bar graph from: Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Primary Care, 37(1); 69-80; 2010.)
To date, prolotherapy has been evaluated using moderate-to-strong assessment techniques and study designs as a treatment for low back pain, osteoarthritis, and several tendinopathies; each condition is a significant cause of pain and disability, and is often refractory to best standard-of-care therapies. The societal impact of these conditions will likely increase in the coming years because the severity and prevalence of each condition is age related. Because the United States population is aging, finding new effective therapies for these conditions can have an impact on individual patient care and overall public health. If effective, the import of nonsurgical interventions such as prolotherapy will also grow. The following section gives an integrated description of higher quality studies assessing prolotherapy for these clinical indications, presented in an order of descending strength of evidence; RCTs are described first (see Table 15-3), and scored using the Delphi scoring system.26 A summary table follows that assigns a formal level of evidence grade for prolotherapy for each condition (Table 15-4).
Table 15-3 Randomized Controlled Trials Assessing Prolotherapy for Tendinopathy, Low Back Pain, and Osteoarthritis
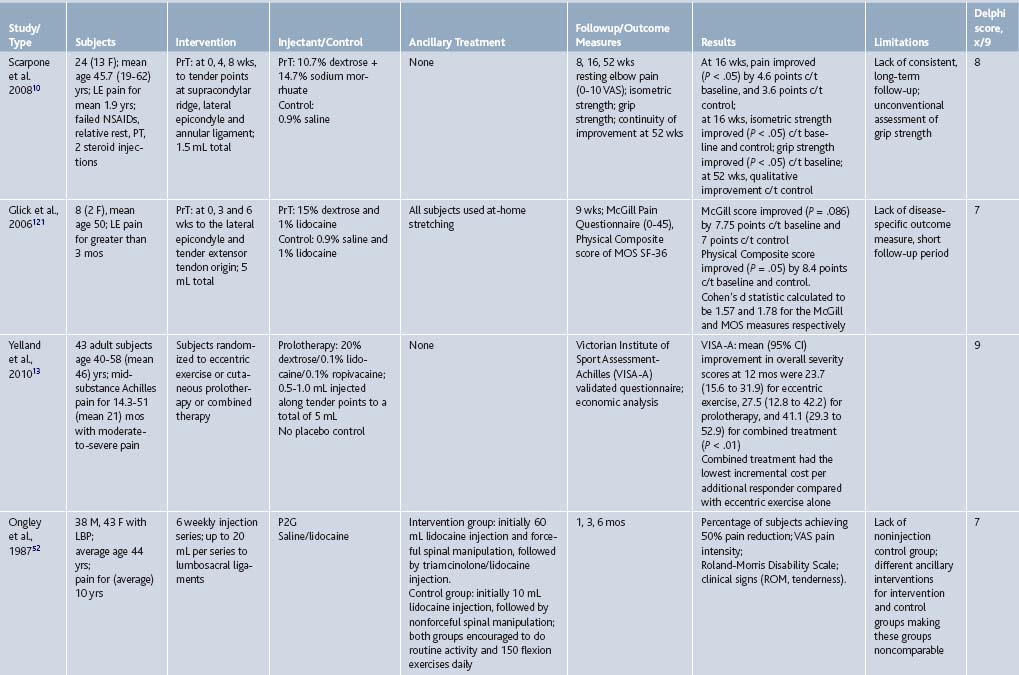
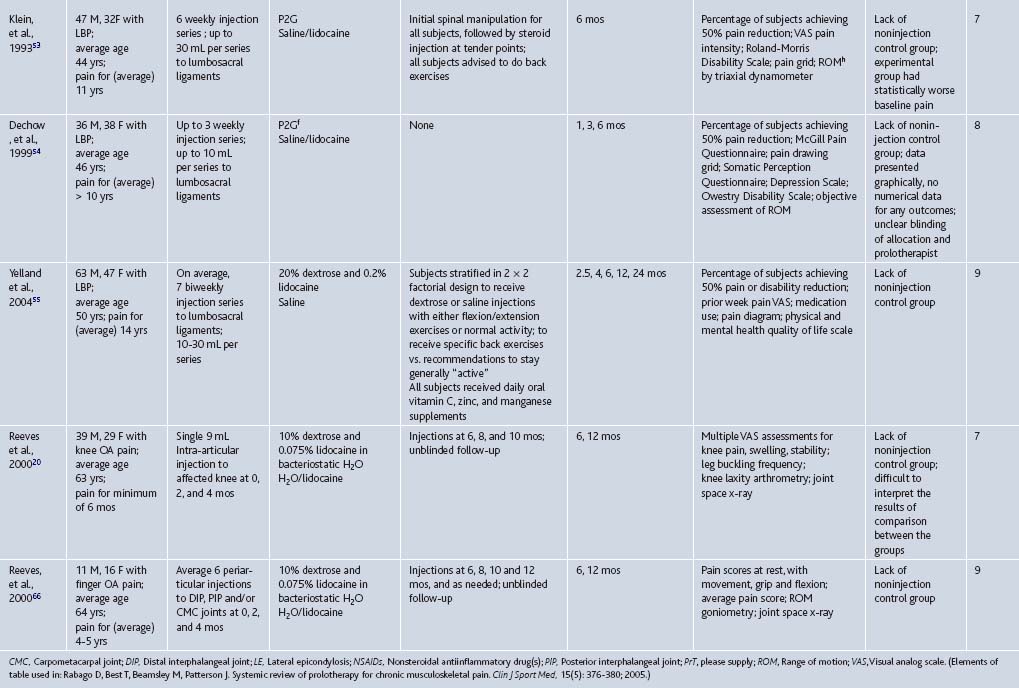
Table 15-4 Strength of Evidence for Prolotherapy as a Treatment for Chronic Musculoskeletal Conditions: Low Back Pain (LBP), Osteoarthritis (OA), and Tendinopathy
| Key Clinical Recommendation on Prolotherapy | Evidence Rating | Reference(s) |
|---|---|---|
| Nonspecific LBP: may be effective; conflicting results in several RCTs | B | 52, 53, 54, 55 |
| Sacroiliac joint dysfunction: may be effective in patients with documented failure of load transfer (disability) at the sacroiliac joint | B | 62 |
| Coccygodynia: may be effective based on prospective case series | B | 63 |
| Lateral epicondylosis: likely effective based on strong positive data in these small RCTs | A | 10 |
| Achilles tendinopathy: may be effective based on high quality prospective case series | B | 7,13 |
| Plantar fasciitis: may be effective, based on high-quality prospective case series | B | 12 |
| B | 20, 66 |
RCTs, Randomized controlled trials.
Tendinopathies
The strongest data supporting the efficacy of prolotherapy for any musculoskeletal condition, compared to control injections, is for chronic, painful overuse tendon conditions that were formerly called “tendonitis” and are now more correctly termed tendinosis or tendinopathy to reflect existing, underlying pathophysiology.27 Tendinopathies are common reasons why patients present to primary care providers and various medical specialists.28,29 Tendinopathies are sometimes discussed as a group because the current understanding of over-use tendinopathies identifies them as sharing underlying non-inflammatory pathology, resulting from a repetitive motion or overuse injury, and associated with painful degenerative tissue. Histopathology of tendon biopsies in patients undergoing surgery for painful tendinopathy reveals collagen separation,30 thin, frayed, and fragile tendon fibrils, separated from each other lengthwise and disrupted in cross-section, increase in tenocytes with myofibroblastic differentiation (tendon repair cells), proteoglycan ground substance, and neovascularization. Classic inflammatory cells are usually absent.30 Although this aspect of tendinosis was first described 25 years ago31 and content experts have advocated a change in nomenclature (from “tendonitis” to tendinosis),27 use of the term “tendonitis” continues.32 Prolotherapy has been assessed as a treatment for four tendon disorders: lateral epicondylosis, hip adductor, Achilles tendinopathies, and plantar fasciitis (see Tables 15-1 and 15-3).
Lateral epicondylosis (LE, “tennis elbow”) is an important common condition of the upper extremity with an incidence of 4 to 7 per 1000 patients per year in primary care settings.33–35 Its greatest impact is on workers with repetitive and high-load upper extremity tasks and on athletes. Its most common cause may be low-load, high-repetition activities such as keyboarding, although formal data are lacking.36 Cost and time away from job or activity are significant.37,38 While many nonsurgical therapies have been tested for LE refractory to conservative measures, none have proved uniformly effective in the long term.39–41 Scarpone and colleagues conducted an RCT to determine whether prolotherapy improves self-reported elbow pain, and objectively measured grip strength and extension strength in patients with chronic LE (see Table 15-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree