 Antibiotic decisions are often more complex in the ICU than in other settings because of an increased risk for unusual infections, a need for consideration of organ failure and extracorporeal devices/drains, as well as a higher risk of poor outcomes, including death, if an incorrect treatment course is chosen.
Antibiotic decisions are often more complex in the ICU than in other settings because of an increased risk for unusual infections, a need for consideration of organ failure and extracorporeal devices/drains, as well as a higher risk of poor outcomes, including death, if an incorrect treatment course is chosen. The need for appropriate cultures cannot be overemphasized. Every attempt should be made to obtain blood cultures prior to initiation of antimicrobial therapy, as well as preantibiotic specimens from other possibly infected sites (e.g., cerebrospinal fluid, urine, lower respiratory tract, pleural fluid). Viral studies and fungal antigen testing should be considered in the appropriate patient.
The need for appropriate cultures cannot be overemphasized. Every attempt should be made to obtain blood cultures prior to initiation of antimicrobial therapy, as well as preantibiotic specimens from other possibly infected sites (e.g., cerebrospinal fluid, urine, lower respiratory tract, pleural fluid). Viral studies and fungal antigen testing should be considered in the appropriate patient. Many adult, and some pediatric, studies have found an association between inappropriate initial empiric antibiotics and increased mortality, increased length of stay, and delayed recovery in sepsis.
Many adult, and some pediatric, studies have found an association between inappropriate initial empiric antibiotics and increased mortality, increased length of stay, and delayed recovery in sepsis. Understanding which organisms are prevalent locally and which antibiotics are most effective against these organisms can enhance antibiotic decisions even after patients’ risk for particular organisms has been determined. Knowledge of local unit and community microbiology can be useful in developing a unit-based ICU empiric antibiotic strategy.
Understanding which organisms are prevalent locally and which antibiotics are most effective against these organisms can enhance antibiotic decisions even after patients’ risk for particular organisms has been determined. Knowledge of local unit and community microbiology can be useful in developing a unit-based ICU empiric antibiotic strategy. In critically ill children with viral respiratory infections, coinfection with bacterial pathogens may occur in 40%-50% of children with respiratory syncytial virus (RSV) bronchiolitis requiring mechanical ventilation. For this reason, cultures, including lower respiratory cultures, are justified from all children requiring intubation and mechanical ventilation for bronchiolitis, along with empiric antibiotic coverage for at least 48-72 hours until culture results return.
In critically ill children with viral respiratory infections, coinfection with bacterial pathogens may occur in 40%-50% of children with respiratory syncytial virus (RSV) bronchiolitis requiring mechanical ventilation. For this reason, cultures, including lower respiratory cultures, are justified from all children requiring intubation and mechanical ventilation for bronchiolitis, along with empiric antibiotic coverage for at least 48-72 hours until culture results return. In general, unless there are compelling reasons to do otherwise, once the pathogen and susceptibilities are known, antimicrobials should be targeted with a spectrum as narrow as possible. Appropriate and improved use of antimicrobials is first and foremost an issue of patient safety and quality.
In general, unless there are compelling reasons to do otherwise, once the pathogen and susceptibilities are known, antimicrobials should be targeted with a spectrum as narrow as possible. Appropriate and improved use of antimicrobials is first and foremost an issue of patient safety and quality. Administering large volumes of fluids, use of extracorporeal support devices, drains, plasmapheresis, and renal replacement therapies can all significantly impact a patient’s volume status and drug distribution. Changes in end-organ function, such as liver, renal, and cardiovascular dysfunction and recovery, can affect drug metabolism and elimination.
Administering large volumes of fluids, use of extracorporeal support devices, drains, plasmapheresis, and renal replacement therapies can all significantly impact a patient’s volume status and drug distribution. Changes in end-organ function, such as liver, renal, and cardiovascular dysfunction and recovery, can affect drug metabolism and elimination. The use of therapeutic drug monitoring should be considered in all drugs in which this is an option.
The use of therapeutic drug monitoring should be considered in all drugs in which this is an option. Effective antibiotic treatment requires more than simply matching a drug with a microorganism. The key is integration of the drug’s unique disposition characteristics in the individual patient (i.e., pharmacokinetics [PK]) with an understanding of the drug’s clinical activity (i.e., pharmacodynamics [PD]).
Effective antibiotic treatment requires more than simply matching a drug with a microorganism. The key is integration of the drug’s unique disposition characteristics in the individual patient (i.e., pharmacokinetics [PK]) with an understanding of the drug’s clinical activity (i.e., pharmacodynamics [PD]). decisions are often more complex in the ICU than in other settings because of an increased risk for unusual infections, a need for consideration of organ failure, the presence of extracorporeal devices, and a higher risk of poor outcomes if an incorrect treatment course is chosen.
decisions are often more complex in the ICU than in other settings because of an increased risk for unusual infections, a need for consideration of organ failure, the presence of extracorporeal devices, and a higher risk of poor outcomes if an incorrect treatment course is chosen. cultures prior to initiation of antimicrobial therapy (1), as well as preantibiotic specimens from other possibly infected sites (e.g., cerebrospinal fluid [CSF], urine, lower respiratory tract, pleural fluid). Viral studies and fungal antigen testing should be considered in the appropriate patient. While a respiratory viral diagnosis may not prompt additional therapy, identification could allow for antibiotic deescalation depending on the clinical context (2,3); however, for critically ill children diagnosed with a viral respiratory infection who do not have airway cultures, the possibility
cultures prior to initiation of antimicrobial therapy (1), as well as preantibiotic specimens from other possibly infected sites (e.g., cerebrospinal fluid [CSF], urine, lower respiratory tract, pleural fluid). Viral studies and fungal antigen testing should be considered in the appropriate patient. While a respiratory viral diagnosis may not prompt additional therapy, identification could allow for antibiotic deescalation depending on the clinical context (2,3); however, for critically ill children diagnosed with a viral respiratory infection who do not have airway cultures, the possibility of a secondary bacterial infection should be considered as coinfection is not uncommon (4).
 studies have found an association between inappropriate initial empiric antibiotics and increased mortality, increased length of stay, and delayed recovery in sepsis. These findings have been seen in global sepsis (8), healthcare-associated MRSA sepsis (9), gram-negative sepsis (10), ventilator-associated pneumonia (VAP) (11), and healthcare-associated pneumonia (HCAP) (12). The HCAP study importantly noted that antibiotic escalation after culture results were obtained still lead to an increased risk of death as compared with those who were prescribed initially appropriate antibiotics. A recent meta-analysis demonstrated significant mortality benefit in sepsis when providing initial appropriate empiric antibiotics, with a number needed to treat (NNT) of 10 patients to prevent 1 death (13). Studies on initial antibiotic appropriateness in critically ill children are lacking, although increased mortality has been associated with inappropriate empiric antibiotics for children with bacteremia in all settings (14) and increased resource utilization in severe community-acquired pneumonia (15).
studies have found an association between inappropriate initial empiric antibiotics and increased mortality, increased length of stay, and delayed recovery in sepsis. These findings have been seen in global sepsis (8), healthcare-associated MRSA sepsis (9), gram-negative sepsis (10), ventilator-associated pneumonia (VAP) (11), and healthcare-associated pneumonia (HCAP) (12). The HCAP study importantly noted that antibiotic escalation after culture results were obtained still lead to an increased risk of death as compared with those who were prescribed initially appropriate antibiotics. A recent meta-analysis demonstrated significant mortality benefit in sepsis when providing initial appropriate empiric antibiotics, with a number needed to treat (NNT) of 10 patients to prevent 1 death (13). Studies on initial antibiotic appropriateness in critically ill children are lacking, although increased mortality has been associated with inappropriate empiric antibiotics for children with bacteremia in all settings (14) and increased resource utilization in severe community-acquired pneumonia (15).
recent hospitalization,
recent antibiotic exposure,
immunosuppression due to disease or medications, and
chronic structural lung disease.
 bacteria identified in their PICU (Fig. 88.1). Understanding which organisms are prevalent locally and which antibiotics are most effective against these organisms can enhance antibiotic decisions even after a patient’s risk for particular organisms has been determined. Knowledge of local unit and community microbiology can be useful in developing a unitbased ICU empiric antibiotic strategy. For example, units with high rates of Stenotrophomonas maltophilia may wish
bacteria identified in their PICU (Fig. 88.1). Understanding which organisms are prevalent locally and which antibiotics are most effective against these organisms can enhance antibiotic decisions even after a patient’s risk for particular organisms has been determined. Knowledge of local unit and community microbiology can be useful in developing a unitbased ICU empiric antibiotic strategy. For example, units with high rates of Stenotrophomonas maltophilia may wish to include coverage of this organism for all patients at risk for healthcare-associated infections.
TABLE 88.1 RISK FACTORS FOR INFECTION DUE TO HEALTHCARE-ASSOCIATED ORGANISMS IN ADULTS | ||
|---|---|---|
|
 requiring mechanical ventilation, coinfection with bacterial pathogens may occur in 40%-50% of cases (4,50,51). Additionally, bacterial coinfections, particularly with S. aureus, are frequently seen in patients with influenza requiring ICU admission (52,53). For these reasons, cultures, including lower respiratory cultures, are justified from all children requiring intubation and mechanical ventilation for viral lower respiratory infections, along with empiric antibiotic coverage until culture results are known (54).
requiring mechanical ventilation, coinfection with bacterial pathogens may occur in 40%-50% of cases (4,50,51). Additionally, bacterial coinfections, particularly with S. aureus, are frequently seen in patients with influenza requiring ICU admission (52,53). For these reasons, cultures, including lower respiratory cultures, are justified from all children requiring intubation and mechanical ventilation for viral lower respiratory infections, along with empiric antibiotic coverage until culture results are known (54). once the pathogen and susceptibilities are known, antimicrobials should be targeted with a spectrum as narrow as possible (55,56).
once the pathogen and susceptibilities are known, antimicrobials should be targeted with a spectrum as narrow as possible (55,56).in fact be harmful (57,58). Consideration of noninfectious etiologies should also occur.
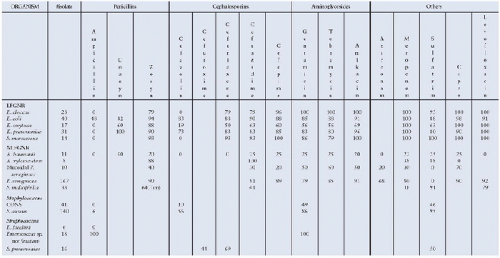 FIGURE 88.1. An excerpt from a PICU-specific antibiogram showing susceptibilities of commonly-isolated organisms to frequently-used antibiotics within one institution. Cipro, ciprofloxacin; Unasyn, ampicillin and sulbactam; Zosyn, piperacillin and tazobactam; LFGNR, lactose-fermenting gram-negative rods; NLFGNR, non-lactose-fermenting gram-negative rods; CONS, coagulase-negative staphylococci. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



