TOPICS
1. What are the systemic implications of pain in the cardiac surgery patient?
2. What modalities are available to treat postoperative pain?
Cardiac surgery can be associated with a significant amount of postoperative pain. Common sources include median sternotomy or thoracotomy incisions, rib retraction, harvesting of vessels from the forearm or legs for grafting, dissection of internal mammary arteries, and the presence of (and removal of) chest drains. Other potential causes include incomplete revascularization of the myocardium, the presence of sternal wires or epicardial pacing leads, and sternocostal and costovertebral pain from excessive retraction.1 While some cardiac surgical techniques confer a greater amount of postoperative pain than others, postoperative pain management should be an anesthetic consideration for all patients undergoing cardiac surgery.
The surgical approach has an obvious impact on the severity of postoperative pain. For example, minimally invasive cardiac procedures such as port access coronary artery bypass (PACAB) and minimally invasive coronary artery bypass (MIDCAB) carry less overall tissue injury and result in less postoperative pain. Postoperative pain for midline sternotomy has often been described to be moderate and patients’ anticipated pain level tends to be much greater than the actual pain they experience postoperatively.2 On the other hand, thoracotomy has been associated with a greater degree of both pain and functional limitation, due to the pain associated with breathing and coughing.3 Endoscopic vein graft harvesting has decreased the severity of postoperative leg pain, as well as the infection and wound dehiscence rate.4
Patient risk factors also play a role in the incidence and severity of post-cardiac surgical pain, with younger patients (< 60 years old) and those with higher New York Heart Association (NYHA) class incurring higher pain scores.5,6
WHAT ARE THE SYSTEMIC IMPLICATIONS OF PAIN IN THE CARDIAC SURGERY PATIENT?
The Stress Response
In addition to the discomfort and suffering that postoperative pain accords, the central nervous system responds to the barrage of noxious afferent impulses with a cascade of neurohumoral responses that impair healing and recovery, and promote poor clinical outcomes. This so-called “stress response” is an adaptive mechanism that serves to liberate fuel through catabolism for energy-dependent fight-or-flight activities, increase blood pressure and heart rate, and promote coagulopathy, inflammation, and immune suppression. This is initiated both through the trauma of the surgical procedure, and uniquely in the case of cardiac surgery, through the use of cardiopulmonary bypass.
Pathophysiologic changes include increased oxygen consumption and energy expenditure; increased secretion of adrenocorticotrophic hormone, cortisol, epinephrine, norepinephrine, insulin, and growth hormone; and decreased total triiodothyronine levels. Quantifiable metabolic consequences of these changes include hyperglycemia, hyperlactatemia, increased free fatty acid concentrations, hypokalemia, increased production of inflammatory cytokines, and increased consumption of complement and adhesion molecules. Cortisol levels can increase to more than 500% of baseline levels and remain elevated for several days.7 Particularly concerning is the rise in catecholamine levels, as these are responsible for much of the cardiovascular morbidity seen postoperatively such as arrhythmias and coronary ischemia.8
Effective attenuation of the entire stress response is not practically achievable, as the effect contributed by cardiopulmonary bypass is difficult to ameliorate. However, management of postoperative pain is a factor within the anesthesiologist’s control. Control of pain by any means will aid in reducing the stress response, but because of the robust nature of the afferent impulses and the duration over which they are delivered to the central nervous system, treatment regimens must be aggressive to have an effect. Typically, this has required the use of multimodal therapies, or the combination of systemic and regional modalities.
Chronic Pain
As our understanding of postoperative pain improves, it has been recognized that acute pain and chronic pain are no longer two separate elements, but are rather two points on a continuum of a pain experience that begins at the time of surgery. As such, many patients will continue to experience pain well after their surgical procedures, eventually manifesting chronic, neuropathic pain that is present after the incisions have healed. Approximately 30% of cardiac patients will develop chronic sternotomy pain lasting 6 months or longer following surgery, and is usually localized to the arms, shoulder, or legs.9 For patients undergoing thoracotomy, chronic pain known as the “post-thoracotomy pain syndrome” is present in approximately 50% of patients, and can lead to disabling chronic neuropathic pain and disability.10
Trauma to peripheral nerves at the time of sternotomy, thoracotomy, or during dissection of the internal mammary artery has been linked to the risk of developing postoperative hyperalgesia or chronic pain; in the latter case, the pain is typically localized to the left sternal border.11
Effective pain management following surgery can possibly aid in the prevention of evolution of acute to chronic pain. For example, patients receiving a single-shot paravertebral block with bupivacaine versus sham block for mastectomy have been shown to have a significantly reduced incidence of chronic incisional pain at 12 months.12 The mechanism for this reduction may relate to the prevention of spinal column recruitment of wide-receptive field neurons, or “wind-up,” a phenomenon that leads to allodynia and hyperalgesia. Several risk factors have been identified as leading to the development of chronic postoperative pain, including psychologic vulnerability, anxiety, and the degree of postoperative pain, all of which are modifiable by a well-planned and executed analgesic plan.13 Indeed, thoracic epidural analgesia has been found to reduce depression and posttraumatic stress disorder following cardiac surgery.14
WHAT MODALITIES ARE AVAILABLE TO TREAT POSTOPERATIVE PAIN?
Systemic Opioids
Intravenous opioids are one of the mainstays of postcardiac analgesia. Ease of administration, predictable effect, and excellent bioavailability are several of the advantages of this method of pain control. Because most postcardiac surgical patients are transferred to an intensely monitored setting and not awakened immediately after surgery, concerns regarding the respiratory depressive effects of opioids are to a large degree ameliorated, at least for the initial several hours until extubation is planned.
Opioid drugs can be naturally occurring (such as morphine or codeine), synthetic (such as fentanyl or tramadol), or semisynthetic (such as hydromorphone). Following cardiac surgery, opioids are typically delivered via either a nurse-controlled (NCA) or patient-controlled opioid analgesia (PCA) regimen. Clearly, to employ the PCA option a patient must be conscious and able to understand the mechanism of pain medication delivery, and for that reason it may not be practical in the immediate postoperative period. However, in a meta-analysis of randomized trials, PCA led to significantly decreased pain scores after cardiac surgery compared to NCA.15 In addition, the cumulative morphine dose was significantly higher with PCA at 24 and 48 hours, suggesting that pain is undertreated in this population when NCA is utilized. Side effects such as respiratory depression and sedation, morbidity and mortality, and ICU and hospital length of stay were not shown to be different between regimens. One randomized controlled trial reported that the incidence of nausea was significantly less with PCA compared with NCA, despite using an overall larger dose of morphine.16 Importantly, the use of a background infusion of morphine when PCA is prescribed increases the total amount of opioid consumed, with little or no clinically relevant improvement in analgesia.17,18
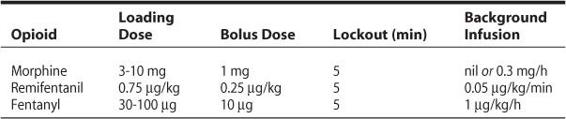
While morphine remains the opioid that most clinicians prescribe following cardiac surgical procedures, other systemic opioids have been investigated for their potential to improve recovery profiles. When compared with PCA morphine, PCA remifentanil was shown to result in significantly reduced pain scores on coughing and movement with remifentanil following coronary artery bypass grafting (CABG).19 Despite this, the group reported excellent analgesia (numeric rating scale < 3) with either regimen overall. A comparison of PCA morphine, fentanyl, and remifentanil following off-pump coronary bypass grafting (OPCAB) demonstrated that the use of remifentanil PCA resulted in similar pain scores as the other opioids, but with less pruritus than fentanyl, and less nausea and vomiting than morphine.20 Because of its ultrashort duration, remifentanil may be particularly useful for avoiding serious side effects such as respiratory depression, as a titrated infusion has been shown to be an effective and safe analgesic regimen in extubated patients after cardiac surgery without resulting in respiratory compromise.21 Suggested PCA opioid regimens are listed in Table 18–1.
Typically, patients will be converted to oral opioid analgesics as early feeding is initiated, based on the usage of parenteral opioids (ie, PCA morphine). This is often done within the first 24 hours after surgery.22 The use of sustained-release opioids such as oxycodone or morphine facilitate a background level of analgesia, and immediate-release versions of the same drugs can be used for breakthrough.
Tramadol is a unique drug that acts both as a mu-opioid agonist, as well as a weak inhibitor of the reuptake of norepinephrine and serotonin, which is thought to enhance analgesia without clinically relevant respiratory depression. It has been well validated as a treatment for mild to moderate postoperative pain, and studies have found equivalency to typical NCA doses of opioids following cardiac surgery.23,24 But et al demonstrated that a single dose of tramadol administered prior to extubation following CABG was associated with a reduction of up to 25% in morphine consumption, as well as a decrease in the visual analog scale (VAS) scores within the first 4 hours postoperatively.25 Because of its effect on serotonin, tramadol use is associated with an increase in postoperative nausea and vomiting, and should not be administered in epileptics.26
Table 18–1. Suggested PCA Regimens for Common Opioids Following Cardiac Surgery
Nonsteroidal Anti-Inflammatory Drugs
NSAIDs have both analgesic and anti-inflammatory properties, and are effective in treating mild to moderate pain when used alone. Combined with other modalities such as opioids, NSAIDs can be used as adjuncts to treat more severe pain, and are useful in reducing opioid requirements.27
NSAIDs inhibit the enzyme cyclooxygenase (COX), thereby decreasing the production of prostaglandins that contribute to inflammation and sensitization of nociceptive fibers. There are two isoforms of the COX enzyme, COX-1 and COX-2. COX-1 is a constitutive enzyme that facilitates the formation of prostaglandins found in blood vessels, stomach, and kidneys and is involved in the preservation of gastric mucosa and maintenance of renal blood flow. In contrast, COX-2 is the inducible isoform, upregulated after tissue injury.
Nonselective NSAIDs have anti-inflammatory and analgesic effects but at the expense of increased gastropathy, decreased renal perfusion, and reduced platelet aggregation.28 Acetylsalicylic acid (ASA) is an example of a nonselective NSAID that the majority of cardiac surgical patients are prescribed specifically for its antiplatelet effects. Controversy surrounding adverse myocardial outcomes in patients taking COX-2 specific inhibitors has led to a great deal of concern over their use in patients at risk for coronary thrombosis. Data demonstrating an association with coronary and cerebral thrombosis resulted in the withdrawal of both rofecoxib and valdecoxib from the US market.29 Parecoxib, an intravenous prodrug of valdecoxib, was issued a letter of non-approval by the FDA in 2005 for similar reasons. Celecoxib, on the other hand, appears to have little effect on coronary thrombosis.30 However, the use of any COX-2 inhibitor in patients with ischemic heart disease should be approached with caution, as other agents are available.
Rapanos et al demonstrated that the combination of indomethacin with morphine after cardiac surgery resulted in reduced postoperative pain scores and opioid use without an increase in NSAID-related side effects.31 Other NSAIDs such as diclofenac and ketoprofen have also been shown to reduce both opioid requirements, opioid-related side effects, and pain scores.32,33 NSAIDs appear to not significantly increase postoperative bleeding or renal dysfunction in postcardiac surgical patients.34
Acetaminophen
Acetaminophen is a common drug for mild to moderate pain. Its favorable safety profile and lack of interactions with other drugs, and absence of any effect on coagulation make it a useful adjunct, with its principal contraindication being patients with hepatic disease. The intravenous form, paracetamol, is in theory ideal for patients after cardiac surgery, who can remain nil by mouth for a day or more following surgery.
Despite the theoretical benefits, data are mixed regarding the overall efficacy of acetaminophen following cardiac surgery, with groups reporting small or no differences with respect to pain scores compared to placebo.35,36 Moreover, a meta-analysis of seven randomized trials showed that the addition of acetaminophen to morphine analgesia after cardiac surgery reduced morphine consumption by 20%, but had no effect on morphine-related side effects or patient satisfaction.37
Thoracic Epidural Analgesia
Thoracic epidural analgesia (TEA) with local anesthetics has been used in cardiac surgery for decades.38 Its benefits include superior analgesia,14 improved pulmonary function,39,40 reduced time to extubation,14,41,42 as well as coronary vasodilation and/or cardioprotection.43–45 It is also the most effective technique by which the stress response is suppressed.46 Liu et al published a meta-analysis of 15 randomized trials and 1178 patients undergoing CABG and found significantly reduced pain scores, time to tracheal extubation, pulmonary complications, and risk of dysrhythmias.47 On the other hand, mortality and myocardial infarction rates were not improved with the use of TEA. However, in a more recent and larger meta-analysis (33 trials, 2366 patients), the composite endpoint of mortality and myocardial infarction was significantly reduced from 5.2% to 2.7%.48 The effect of TEA on incidence of atrial fibrillation is unclear; while it was not shown to be reduced for on-pump cardiac surgery,49 a significant reduction was found in off-pump coronary surgery (23.7% vs 3%).50
The technique’s beneficial effects can be explained by the halting of afferent neural input from the surgical site to the spinal cord and higher centers in the central nervous system. This not only provides excellent analgesia, but also prevents the initiation of the stress response, which limits the release of catecholamines and counter-regulatory hormones and attenuates the hypercoagulable state that might otherwise predispose to thrombotic events postoperatively. In addition, sympathetic blockade of spinal levels T1 to T4 by TEA can block cardiac afferent (thereby reducing anginal symptoms) and efferent fibers (promoting coronary vasodilation and reducing heart rate and left ventricular work index). Jakobsen et al conducted 2-D echocardiograms in patients with ischemic heart disease and found that TEA resulted in improved left ventricular systolic and diastolic function, likely mediated through improved cardiac loading conditions.51 Note that for the thoracic epidural to be of maximum benefit, it should be placed at the level of T3/T4, in order to best provide analgesia to the sternal area (T2-T5), as well as provide sympathetic blockade of the cardioaccelerator fibers (T1-T4). The skin overlying the superior aspect of the sternum and manubrium is not covered by thoracic nerves, but is instead innervated by the supraclavicular nerve (C3/C4), a branch of the cervical plexus. This is effectively supplemented with a lateral subcutaneous field block at the level of the clavicular heads.
Despite its attractive theoretical benefits, the use of TEA is controversial and is not widespread. The greatest barrier to its implementation is likely the fear of epidural hematoma associated with anticoagulation for cardiopulmonary bypass. Recent estimation of this risk is approximately 1:12,000 procedures performed, with 95% confidence intervals of 1:2100 to 1:68,000.52 In order to reduce the risk of incurring a hematoma, most publications advise placing the epidural the evening prior to surgery. This practice, however, is clearly impractical in an age of same-day admissions. Indeed, there is no evidence that insertion the night before surgery reduces the risk compared to insertion on the day of surgery.53 Practically speaking, epidural catheters should be placed as early as possible on the morning of surgery (ie, after the intravenous line is inserted), and only in patients who have a normal coagulation profile at baseline. This ensures at least 1 hour prior to anticoagulation, which is consistent with the recommendations of the American Society of Regional Anesthesia Consensus Conference on Neuraxial Anesthesia and Anticoagulation.54 The smallest amount of heparin and protamine should be employed, and the patient monitored closely for signs and symptoms of an expanding hematoma. Difficulty arises when a traumatic tap is encountered. The Consensus Conference recommends a delay of 24 hours to ensure that complete clotting of the epidural vein has occurred. Clearly, these issues must be discussed with the patient prior to the procedure during the informed consent. The postoperative local anesthetic infusion should consist of the lowest concentration possible that provides analgesia, but not motor block (eg, ropivacaine 0.15%-0.2%), to help aid in the possible diagnosis of epidural hematoma, should increasing motor weakness be noted.
Intrathecal Opioids
Another regional analgesic option for postcardiac surgery is intrathecal opioids. Morphine is by far the drug studied best, by virtue of its ability to confer a long (eg, 24 hours) period of quality analgesia. Various doses (250-4000 μg) have been used, with larger doses likely to prolong the time to extubation due to the respiratory depressive effects.55 This is usually a consideration only if the patients are to be fast-tracked.
A meta-analysis of 17 trials enrolling 668 patients demonstrated little difference in important outcomes between those randomized to receive intrathecal opioids or not; however, the incidence of pruritus was significantly higher in the intervention group.47 Moreover, a recent meta-analysis of 1106 patients receiving general anesthesia with or without spinal opioids showed no difference in mortality, myocardial infarction, or length of hospital stay.56 The addition of 100 μg of clonidine to 500 μg of spinal morphine appears to decrease postoperative pain scores and time to extubation.57
While the risk of epidural hematoma is rare (and probably lower than that of an epidural), the true incidence is unknown, and discussion of the potential for this complication with the patient is warranted. In general, this technique has fallen out of favor due to the lack of substantial benefit, and the potential for delaying fast-track extubation.
Regional Blocks and Cardiac Surgery
Concern over epidural hematoma formation with TEA has led some practitioners to consider alternative regional methods for postoperative pain control such as intercostal and paravertebral blocks.
Intercostal nerve (IC) blocks are simple to perform and, if properly performed, result in excellent analgesia of the chest and upper abdominal wall. Typically, these blocks are used for thoracotomy or minithoracotomy incisions. Disadvantages include the potential for pleural, lung parenchymal, or vascular puncture, and the need to perform bilateral blockade if the patient’s incision is midline. In addition, IC blocks do not block visceral pleural pain, as the sympathetic nerves are not blocked. Traditionally, IC blocks are performed at the angle of the rib, which is impractical in an intubated postcardiac surgical patient. Instead, bilateral IC blockade with parasternal catheters has been employed by the surgeon effectively after cardiac surgery, reducing morphine requirements and hospital length of stay.58
Paravertebral block (PVB) involves injection of local anesthetic in the paravertebral space immediately lateral to the place from where the spinal nerves emerge from the intervertebral foramina. It is typically performed prior to inducing general anesthesia for cardiac surgery, and excellent analgesia can be achieved with infusion catheters located on either side of the midline. Advantages over the IC block include more complete analgesia of the posterior chest wall, and sympathetic block of the involved levels, which aids in visceral pain and provides cardiac sympathectomy. PVB has been found to be as effective for analgesia as TEA for MIDCAB.59
Disadvantages include the potential for needle misadventure and subsequent pneumothorax or neuraxial placement of the needle. Also, 10% of patients exhibit parasympathetic reactions at needle placement resulting in hypotension, bradycardia, and near syncope. This technique may also be a problem in patients with spinal anomalies, trauma, or a history of spine surgery. Recently, an ultrasound-guided technique of continuous paravertebral block using a more lateral intercostal approach has been described to potentially minimize the risk of vascular puncture, nerve injury, and pneumothorax.60 This technique takes advantage of the continuity that exists between the intercostal and paravertebral spaces to provide a method of continuous paravertebral block that may provide advantages when compared with the classical approach for the continuous paravertebral nerve block.
PVB has been used extensively for thoracotomy, and has been shown in a systematic review to be as effective as epidural analgesia for pain management following thoracotomy.61 However, the side-effect profile appears to favor PVB over TEA for this population. Pulmonary function assessed by peak expiratory flow rate was significantly better preserved in the paravertebral group. In addition, epidural block was associated with a higher incidence of urinary retention, nausea, pruritus, and hypotension. Finally, epidurals were associated with delayed operative start time, and a higher rate of technical failure and displacement.
Efforts have been made to simplify some of these regional approaches by infusing local anesthetic directly into the fascial plane of the sternotomy wound by catheters. Results have been mixed, however, with studies showing both a favorable and no effect on pain scores following cardiac surgery.62,63
SUMMARY
Cardiac surgery can lead to a significant degree of postoperative pain that has traditionally been managed with intravenous opioids. The nowadays trends toward fast tracking of the patients after cardiac surgery have made careful planning of postoperative analgesia an even more important concern. Multimodal therapeutic plans using a variety of agents can be customized to meet the needs of a specific patient. In particular, the use of patient controlled analgesia with short-acting opioids is an effective way by which patients can titrate their own pain management; a background of analgesia created by the administration of systemic non-opioids such as tramadol, acetaminophen, and/or NSAIDs ensures that alterations in pain intensity are met with a sustained level of comfort.
Thoracic epidural analgesia has been shown to improve a variety of important outcomes, including the composite endpoint of mortality and myocardial infarction. Despite this, concern regarding the potential for epidural hematoma has limited its use, regardless of evidence pointing to its overall safety. This trend may change in the future as more evidence of the technique’s favorable effects come to light. Alternatively, other regional anesthesia techniques such as intercostal and paravertebral blocks are proven methods which reduce post-sternotomy and thoracotomy pain and improve the recovery profile for this population of patients.
REFERENCES
1. Alston RP, Pechon P. Dysaesthesia associated with sternotomy for heart surgery. Br J Anaesth. 2005;95:153-158.
2. Nay PG, Elliott SM, Harrop-Griffiths AW. Postoperative pain. Expectation and experience after coronary artery bypass grafting. Anaesthesia. 1996;51:741-743.
3. Diegeler A, Walther T, Metz S, et al. Comparison of MIDCAP versus conventional CABG surgery regarding pain and quality of life. Heart Surg Forum. 1999;2:290-295; discussion 295-296.
4. Andreasen JJ, Nekrasas V, Dethlefsen C. Endoscopic vs open saphenous vein harvest for coronary artery bypass grafting: a prospective randomized trial. Eur J Cardiothorac Surg. 2008;34:384-389.
5. Kalso E, Mennander S, Tasmuth T, Nilsson E. Chronic post-sternotomy pain. Acta Anaesthesiol Scand. 2001;45:935-939.
6. Mueller XM, Tinguely F, Tevaearai HT, Revelly JP, Chiolero R, von Segesser LK. Pain location, distribution, and intensity after cardiac surgery. Chest. 2000;118:391-396.
7. Hoda MR, El-Achkar H, Schmitz E, Scheffold T, Vetter HO, De Simone R. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2179-2186.
8. Riles TS, Fisher FS, Schaefer S, Pasternack PF, Baumann FG. Plasma catecholamine concentrations during abdominal aortic aneurysm surgery: the link to perioperative myocardial ischemia. Ann Vasc Surg. 1993;7:213-219.
9. Meyerson J, Thelin S, Gordh T, Karlsten R. The incidence of chronic post-sternotomy pain after cardiac surgery—a prospective study. Acta Anaesthesiol Scand. 2001;45:940-944.
10. Karmakar MK, Ho AM. Post-thoracotomy pain syndrome. Thorac Surg Clin. 2004;14:345-352.
11. Eisenberg E, Pultorak Y, Pud D, Bar-El Y. Prevalence and characteristics of post coronary artery bypass graft surgery pain (PCP). Pain. 2001;92:11-17.
12. Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103:703-708.
13. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123-1133.
14. Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. 2003;75:93-100.
15. Bainbridge D, Martin JE, Cheng DC. Patient-controlled versus nurse-controlled analgesia after cardiac surgery—a meta-analysis. Can J Anaesth. 2006;53:492-499.
16. O’Halloran P, Brown R. Patient-controlled analgesia compared with nurse-controlled infusion analgesia after heart surgery. Intensive Crit Care Nurs. 1997;13:126-129.
17. Dal D, Kanbak M, Caglar M, Aypar U. A background infusion of morphine does not enhance postoperative analgesia after cardiac surgery. Can J Anaesth. 2003;50:476-479.
18. Guler T, Unlugenc H, Gundogan Z, Ozalevli M, Balcioglu O, Topcuoglu MS. A background infusion of morphine enhances patient-controlled analgesia after cardiac surgery. Can J Anaesth. 2004;51:718-722.
19. Baltali S, Turkoz A, Bozdogan N, et al. The efficacy of intravenous patient-controlled remifentanil versus morphine anesthesia after coronary artery surgery. J Cardiothorac Vasc Anesth. 2009;23:170-174.
20. Gurbet A, Goren S, Sahin S, Uckunkaya N, Korfali G. Comparison of analgesic effects of morphine, fentanyl, and remifentanil with intravenous patient-controlled analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:755-758.
21. Steinlechner B, Koinig H, Grubhofer G, et al. Postoperative analgesia with remifentanil in patients undergoing cardiac surgery. Anesth Analg. 2005;100:1230-1235, table of contents.
22. Kogan A, Medalion B, Raanani E, et al. Early oral analgesia after fast-track cardiac anesthesia. Can J Anaesth. 2007;54:254-261.
23. Manji M RC, Jones P, Ariffin S, Faroqui M. Tramadol for postoperative analgesia in coronary artery bypass graft surgery [abstract]. Br J Anaesth. 1997;78:A87.
24. Sellin MLV, Sicsic JC. Postoperative pain: tramadol vs morphine after cardiac surgery [abstract]. Br J Anaesth. 1998;80:41.
25. But AK, Erdil F, Yucel A, Gedik E, Durmus M, Ersoy MO. The effects of single-dose tramadol on postoperative pain and morphine requirements after coronary artery bypass surgery. Acta Anaesthesiol Scand. 2007;51:601-606.
26. Desmeules JA. The tramadol option. Eur J Pain. 2000;4 (suppl A):15-21.
27. Tramer MR, Williams JE, Carroll D, Wiffen PJ, Moore RA, McQuay HJ. Comparing analgesic efficacy of non-steroidal anti-inflammatory drugs given by different routes in acute and chronic pain: a qualitative systematic review. Acta Anaesthesiol Scand. 1998;42:71-79.
28. Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26:2-10.
29. Sanghi S, MacLaughlin EJ, Jewell CW, et al. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc Hematol Disord Drug Targets. 2006;6:85-100.
30. Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59 (suppl 2):117-133.
31. Rapanos T, Murphy P, Szalai JP, Burlacoff L, Lam-McCulloch J, Kay J. Rectal indomethacin reduces postoperative pain and morphine use after cardiac surgery. Can J Anaesth. 1999;46:725-730.
32. Dhawan N, Das S, Kiran U, Chauhan S, Bisoi AK, Makhija N. Effect of rectal diclofenac in reducing postoperative pain and rescue analgesia requirement after cardiac surgery. Pain Pract. 2009;9:385-393.
33. Hynninen MS, Cheng DC, Hossain I, et al. Non-steroidal anti-inflammatory drugs in treatment of postoperative pain after cardiac surgery. Can J Anaesth. 2000;47:1182-1187.
34. Kulik A, Ruel M, Bourke ME, et al. Postoperative naproxen after coronary artery bypass surgery: a double-blind randomized controlled trial. Eur J Cardiothorac Surg. 2004;26:694-700.
35. Lahtinen P, Kokki H, Hendolin H, Hakala T, Hynynen M. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesth Analg. 2002;95:813-819, table of contents.
36. Cattabriga I, Pacini D, Lamazza G, et al. Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: a double blind randomized controlled trial. Eur J Cardiothorac Surg. 2007;32:527-531.
37. Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505-513.
38. Robinson RJ, Brister S, Jones E, Quigly M. Epidural meperidine analgesia after cardiac surgery. Can Anaesth Soc J. 1986;33:550-555.
39. Scott NB, Turfrey DJ, Ray DA, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528-535.
40. Groeben H. Epidural anesthesia and pulmonary function. J Anesth. 2006;20:290-299.
41. Priestley MC, Cope L, Halliwell R, et al. Thoracic epidural anesthesia for cardiac surgery: the effects on tracheal intubation time and length of hospital stay. Anesth Analg. 2002;94:275-282, table of contents.
42. Barrington MJ, Kluger R, Watson R, Scott DA, Harris KJ. Epidural anesthesia for coronary artery bypass surgery compared with general anesthesia alone does not reduce biochemical markers of myocardial damage. Anesth Analg. 2005;100:921-928.
43. Berendes E, Schmidt C, Van Aken H, et al. Reversible cardiac sympathectomy by high thoracic epidural anesthesia improves regional left ventricular function in patients undergoing coronary artery bypass grafting: a randomized trial. Arch Surg. 2003;138:1283-1290, discussion 1291.
44. Blomberg S, Emanuelsson H, Kvist H, et al. Effects of thoracic epidural anesthesia on coronary arteries and arterioles in patients with coronary artery disease. Anesthesiology. 1990;73:840-847.
45. Kock M, Blomberg S, Emanuelsson H, Lomsky M, Stromblad SO, Ricksten SE. Thoracic epidural anesthesia improves global and regional left ventricular function during stress-induced myocardial ischemia in patients with coronary artery disease. Anesth Analg. 1990;71:625-630.
46. Loick HM, Schmidt C, Van Aken H, Junker R, Erren M, Berendes E, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg. 1999;88:701-709.
47. Liu SS, Block BM, Wu CL. Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta-analysis. Anesthesiology. 2004;101:153-161.
48. Bignami E, Landoni G, Biondi-Zoccai GG, et al. Epidural analgesia improves outcome in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2009;24(4):586-597.
49. Tenenbein PK, Debrouwere R, Maguire D, et al. Thoracic epidural analgesia improves pulmonary function in patients undergoing cardiac surgery. Can J Anaesth. 2008;55:344-350.
50. Bakhtiary F, Therapidis P, Dzemali O, et al. Impact of high thoracic epidural anesthesia on incidence of perioperative atrial fibrillation in off-pump coronary bypass grafting: a prospective randomized study. J Thorac Cardiovasc Surg. 2007;134:460-464.
51. Jakobsen CJ, Nygaard E, Norrild K, et al. High thoracic epidural analgesia improves left ventricular function in patients with ischemic heart. Acta Anaesthesiol Scand. 2009;53:559-564.
52. Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: an updated risk assessment. Heart Surg Forum. 2007;10:E334-E337.
53. Chaney MA. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesth Analg. 2006;102:45-64.
54. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003;28:172-197.
55. Konstantatos A, Silvers AJ, Myles PS. Analgesia best practice after cardiac surgery. Anesthesiol Clin. 2008;26:591-602.
56. Zangrillo A, Bignami E, Biondi-Zoccai GG, et al. Spinal analgesia in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2009;23:813-821.
57. Nader ND, Li CM, Dosluoglu HH, Ignatowski TA, Spengler RN. Adjuvant therapy with intrathecal clonidine improves postoperative pain in patients undergoing coronary artery bypass graft. Clin J Pain. 2009;25:101-106.
58. McDonald SB, Jacobsohn E, Kopacz DJ, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. 2005;100:25-32.
59. Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288-292.
60. Ben-Ari A, Moreno M, Chelly JE, Bigeleisen PE. Ultrasound-guided paravertebral block using an intercostal approach. Anesth Analg. 2009;109:1691-1694.
61. Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact Cardiovasc Thorac Surg. 2010;10:92-96.
62. Magnano D, Montalbano R, Lamarra M, et al. Ineffectiveness of local wound anesthesia to reduce postoperative pain after median sternotomy. J Card Surg. 2005;20:314-318.
63. White PF, Rawal S, Latham P, et al. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology. 2003;99:918-923.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








