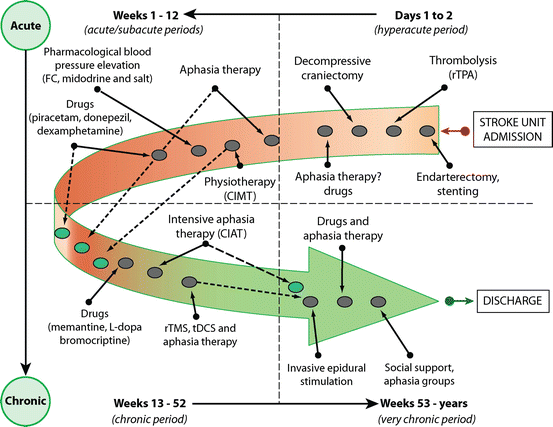
Fig. 6.1
Algorithm for different types of aphasia based on repetition ability coupled with performance on fluency in spontaneous speech and auditory comprehension. Adapted from [61]
Six of the eight acute clinical aphasic syndromes can be broadly classified in two groups. Features common to one such group, usually described as “classic” aphasias (e.g., Broca’s aphasia, Wernicke aphasia, and conduction aphasia), are impaired repetition and cortical involvement of the perisylvian language region irrigated by the middle cerebral artery [23, 24, 27, 40, 41]. Features characteristic of the other group (e.g., transcortical aphasias) are preserved repetition and cortical damage at or beyond the boundaries of the perisylvian language region [23, 24, 27, 34, 40, 41]. The cortical areas residing outside the perisylvian cortex are perfused by the anterior cerebral artery, the posterior cerebral artery, and the farthest end branches of two adjacent vascular territories [23, 24, 27, 34, 40, 41]. Of the remaining two aphasic syndromes, one (e.g., global aphasia) presents with impaired repetition, whereas repetition is preserved in the other (e.g., anomic aphasia). These two aphasias lack a consistent localizing value because they are associated with damage to different lesion sites [27].
Broca’s Aphasia
Broca’s aphasia is characterized by nonfluent spontaneous speech, naming, and repetition with relative sparing of auditory and reading comprehension except for syntactically complex sentences [23, 24, 27, 40, 41]. Nonfluent speech is characterized by reduced phrase length and word production (<50 words minute). Moreover, speech quality is also impaired by the presence of hesitant and effortful emissions, which contain abnormal rhythm, stress, and intonation. Grammar is impoverished (agrammatism) and many patients additionally display inconsistent articulation errors on repeated speech productions of the same utterance (apraxia of speech). Language comprehension is preserved for conversational, word-recognition tasks and simple commands, but understanding of syntactically complex sentences is abnormal. Repetition is impaired particularly for long words and short phrases, but sometimes is better than spontaneous speech. Naming is abnormal, but objects (nouns) are better named than actions (verbs). Oral reading and writing are grossly impaired. Patients with Broca’s aphasia usually display depression and catastrophic reactions (e.g., outbursts of frustration and anger when confronted with a task) [49, 50]. Lesions responsible for Broca’s aphasia are not restricted to the left frontal operculum; they usually exceed the boundaries of Broca’s area extending deeply to lower motor cortex, temporal cortex, anterior insula, basal ganglia, and internal capsule and other white matter tracts [42].
Wernicke’s Aphasia
Wernicke’s aphasia is characterized by fluent speech with impaired comprehension and repetition [23, 24, 27, 40, 41]. Spontaneous speech is fluent, well-articulated, and melodic, but marred by the presence of neologisms, phonemic, and, less frequently, semantic paraphasias. Oral production may be abundant, copious, and sometimes adopt features of logorrhea (incoherent talkativeness) and pressure of speech, giving rise to an unintelligible jargon. Disinhibition of speech results, at least in part, from reduced self-monitoring of discourse, insight, and behavioral control (elation, irritability) [8, 49]. Repetition, naming, reading, and writing are grossly disrupted. Wernicke’s aphasia is associated with large posterior cortical lesions involving the posterior temporal gyrus and neighboring parietal or occipital cortical areas [42].
Conduction Aphasia
Conduction aphasia is characterized by a disproportionate deficit in repetition in the context of fluent paraphasic verbal output and relative sparing of auditory comprehension [23, 24, 27, 40, 41]. Two main types of conduction aphasia have been described—reproduction and repetition [51]. The reproduction subtype is characterized by phonemic paraphasias in all verbal domains and recurrent production of sequential phonemic approximations to self-repair errors (conduite d’approche) [52, 53]. The reproduction conduction aphasia variety has been variously attributed to deficits in verbal praxis [54], disrupted speech programming [55], poor output phonological encoding [55, 56], or a combination of abnormal sensory-motor integration and reduced phonological short-term memory [57, 58], which may result from cortical damage without necessary compromise of the arcuate fasciculus. The repetition subtype shows virtually isolated repetition deficits that have been linked to a selective impairment in auditory-verbal short-term memory and cortical damage that extend deeply to affect the arcuate fasciculus [52, 53, 56].
Global Aphasia
Global aphasia is a severe condition that combines the deficits described in Broca’s and Wernicke’s aphasia [1, 23, 24, 27]. Thus, a grave reduction of verbal fluency (usually limited to word perseverations (e.g., “pa..pa..pa..pa”)) coexists with severely impaired comprehension. Repetition, naming, reading, and writing are also profoundly impaired. As previously mentioned, acute global aphasia does not have a consistent localizing value because, although it usually results from involvement of both Broca’s and Wernicke’s area, large acute ischemic or hemorrhagic lesions in either cortical area can also produce the syndrome [27].
Transcortical Aphasia
Transcortical aphasia is the term used for syndromes in which the ability to repeat language is relatively preserved despite marked disturbances in other linguistic domains [34]. The transcortical syndromes are relatively similar to those describe previously, except for the preservation of repetition performance and a tendency to repeat words and utterances spoken by another person (echolalia) [24, 34]. Repetition is relatively preserved in the face of deficits in spontaneous speech (transcortical motor aphasia), auditory comprehension (transcortical sensory aphasia), or both (mixed transcortical aphasia) [34]. All clinical variants of transcortical aphasias have been attributed to lesions that spare the left perisylvian area and white matter tracts including the arcuate fasciculus. The most likely mechanism underpinning transcortical syndromes is the so-called isolation of speech area, which proposes that vascular lesions in watershed territories between major cerebral arteries or in the anterior cerebral artery and the posterior cerebral artery (extrasylvian cortical areas) disrupt the connectivity between frontal areas responsible for speech initiation or posterior conceptual-semantic representation areas and the intact perisylvian language core and its underlying white matter fiber pathways [23, 24, 34, 40]. Anterior lesions in watershed territories or in the anterior cerebral artery territory cause the motor variant, posterior lesions are responsible for the sensory type, and damage to both anterior and posterior watershed territories are associated with the mixed form.
Anomic Aphasia
Anomic aphasia is characterized by the inability to produce names in spontaneous speech and visual confrontation naming [23, 27]. Anomia is present in all types of aphasias, to the extent that the diagnosis of aphasia is uncertain in language-impaired patients showing preserved naming ability. Acute anomic aphasia is rare and may indicate damage to the left temporal cortex, whereas in chronic stroke, anomic aphasia is commonly the final classification for patients evolving from more severe fluent and nonfluent aphasias [58, 59].
Assessment: A Multidimensional Approach
A comprehensive evaluation of language deficits in PSA is essential for establishing neuroscientifically based recommendations for clinical practice of aphasia rehabilitation [1, 60, 61]. The evaluation of language deficits should be flexible and adjusted to the general, neurological, and functional status of the aphasic patient over the different phases of stroke recovery. For instance, in the early stages (i.e., hyperacute and acute periods) bedside language testing is preferable because most aphasic patients do not tolerate lengthy evaluations, whereas more in-depth evaluations are feasible in chronic patients. One simple manner to identify aphasia in the emergency room, particularly by clinicians unfamiliar with the assessment of language disorders, is testing spontaneous speech during an informal conversation or asking patients to write a sentence [61] (see Fig. 6.1). Aphasia is nearly always accompanied by deficits in spontaneous speech and writing, so that if the patient fails to do these simple tasks and commits a number of errors in both output modalities, the presence of aphasia is highly probable. Once the diagnosis of aphasia is established, it is necessary to assess the clinical profile and severity of language deficits for two important reasons. First, recent research has demonstrated that the initial aphasia severity is the best predictor of long-term outcome [62]. Second, data on aphasia type and severity are useful for managing recovery, devising tailor-made interventions, and informing the patient and relatives about prognosis as well as therapeutic possibilities [1].
An important aspect of the evaluation concerns the use of standardized aphasia tests. Figure 6.2 shows a diagram that can guide clinicians to determine the best assessment tools and timing for implementing different strategies of evaluation. In the acute PSA stage, testing of aphasic patients is often limited by short attention span, distractibility, fatigability, and lack of cooperation and other nonlanguage deficits affecting executive function and visual perception [59, 63–65]. Therefore, a bedside screening of language deficits using standardized scales (e.g., Mississippi Aphasia Screening Test, Frenchay Aphasia Screening Test) [66, 67] is recommended. These screening tests are not time-consuming and can be applied even in severely impaired patients. In less affected patients or in more chronic patients, a comprehensive evaluation is required. This can be done with standardized batteries (e.g., Western Aphasia Battery and Boston Diagnostic Aphasia Examination) [41, 68] that combine data from different language subtests (e.g., spontaneous, speech, auditory comprehension, repetition, and naming) to achieve a profile of aphasia (i.e., Broca’s, Wernicke’s, and others) and composite scores of aphasia severity (e.g., Aphasia Quotient of the Western Aphasia Battery) [41]. The individual test scores and composite scores of these batteries are useful to estimate longitudinal changes in the pattern and overall severity of aphasia occurring spontaneously or promoted by speech-language therapy (SLT) and other therapeutic interventions [64, 65]. Nevertheless, to plan rational and individualized SLT interventions, a more in-depth language evaluation in the frame of the cognitive neuropsychological approach is necessary. Tests like the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA) [69] are particularly useful for identifying deficits in discrete cognitive systems as well as residual areas of strength that can be useful in the service of language recovery.
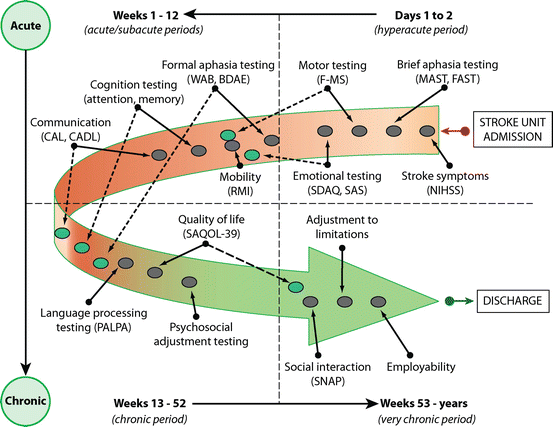

Fig. 6.2
The diagram shows the different post-stroke aphasia stages and testing tools, which can guide clinicians to select the appropriate tool for a specific function. NIHSS National Institutes of Health Stroke Scale [13], F-MS Fugl-Meyer Scale [111], MAST Mississippi Aphasia Screening Test, FAST Frenchay Aphasia Screening Test [66, 67], SAQOL-39 Aphasia Quality of Life Scale-39 [112], SDAQ stroke aphasic depression questionnaire [113], SAS Starkstein’s Apathy Scale [8, 9], WAB western aphasia battery [41], BDAE Boston Diagnostic Aphasia Examination [68], PALPA Psycholinguistic Assessment of Language Processing in Aphasia [69], CAL communicative activity log [98], SNAP social network with aphasia profile [114], RMI rivermead mobility index [115]. Continuous lines and gray circles indicate tasks that are preferentially administered in the period, whereas dotted lines and turquoise circles indicate tasks that may be administered in different aphasia periods
The aforementioned cognitive impairments in aphasic patients go beyond language deficits to affect other cognitive domains (e.g., attention, visuospatial working memory, processing speed, learning and memory, and executive function) essential for normal language functioning. Because the functioning of these nonlinguistic cognitive functions can predict recovery from language and communication deficits in PSA [5, 70, 71], formal assessment of aphasia should be expanded to other cognitive and noncognitive domains (e.g., mood and motivation). The rational manner to implement this methodology is by using a multidimensional approach [1]. In the past few years, the formal assessment of PSA has progressed from the evaluation of language deficits to a more comprehensive evaluation that includes testing of verbal and nonverbal communication, aphasia-related cognitive deficits (e.g., attention, executive function, and memory), mood and motivation, activities of daily living, social interaction, and the impact of aphasia on relatives and careers (see Fig. 6.2).
Prognosis and Mechanisms of Recovery
Recovery is an ongoing process and most patients continue to make progress several years after PSA onset. The greatest spontaneous recovery occurs in the first 2 or 3 months [1]. Some studies have claimed that prognosis for full recovery in the hyperacute period is good (>70 % by 6 months) particularly for patients suffering mild strokes and when aphasia is not associated with other neurologic deficits [72]. However, in some studies reporting good prognosis, language assessment carried out with testing tools (e.g., National Institutes of Health Stroke Scale) was unable to capture residual language deficits [13, 72]. In the remaining patients, the amount of improvement was less detectable in the following months, and most of them reached a plateau after 1 year [1]. Around 40 % of PSA patients showed complete recovery within 1 year. The type of aphasia always evolves to a less severe form during the first year and only certain aphasic patterns (i.e., anomic, Broca’s, and conduction) prevail in chronic phases [19, 73, 74]. Figure 6.3 shows the possible patterns of recovery in PSA evolving from acute global aphasia to less severe aphasic syndromes. Similar patterns of recovery can also be observed in other acute aphasic syndromes (e.g., Wernicke’s aphasia evolving to less severe conduction aphasia). By contrast, sometimes PSA worsens, indicating the occurrence of a new lesion, the aggravation of previous cognitive failure, or the development of post-stroke dementia in elderly patients [64, 75].
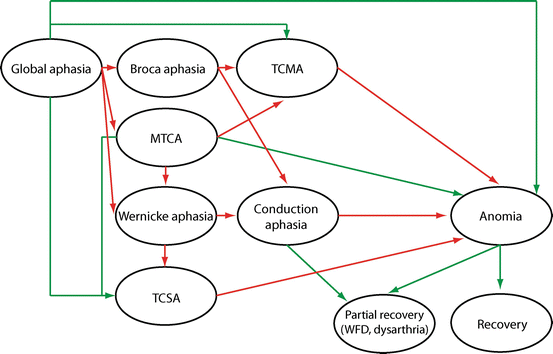

Fig. 6.3
Possible patterns of recovery in PSA evolving from acute global aphasia to less severe aphasic syndromes. Red lines show the usual transitions from one aphasic to others less severe following a logical sequence of resolving language deficits. For instance, improvement of auditory comprehension converts global aphasia in Broca’s aphasia, and further recovery of repetition transforms Broca’s aphasia in TCMA (transcortical motor aphasia). By contrast, green lines show possible, but less frequent, evolution of aphasic syndromes. Acute aphasic syndromes showing rapid resolution, as take place in hemorrhages, often circumvent the logical sequence of aphasia resolution, thus rapidly converting a severe aphasia to a very less severe syndrome or to partial/complete recovery. MTCA mixed transcortical aphasia, TCSA transcortical sensory aphasia, WFD word-finding difficulty
The mechanisms underlying recovery from PSA include restoration of cerebral blood flow in the area of the ischemic penumbra (a highly vulnerable peri-infarct tissue that may survive or die), release of remote cortical and subcortical areas from initial inhibition (diaschisis), dynamic network changes, unmasking of preexisting connections, activity-dependent synaptic changes, and restoration of neurotransmitter activity [76–78]. Restorative mechanisms may operate with different timetables, and their availability may depend upon the site and extent of the lesion as well as on the residual capacity of partially damaged and non-damaged neural tissue to ameliorate abnormal language functions [62, 79–82]. In this regard, the integrity of certain cortical areas (e.g., left hippocampus) [83] or white matter pathways (e.g., subcallosal fasciculus and arcuate fasciculus) [84, 85] seems to be crucial for recovering speech production in patients with large infarctions in the left middle cerebral artery (Fig. 6.4). On the other hand, PSA cases associated with infarcts in arterial border-zone areas between the left middle cerebral artery and either the anterior cerebral artery or the posterior cerebral artery territories sparing the perisylvian cortex show better long-term prognosis [86, 87]. Recovery also relies on the interplay between patient’s characteristics (i.e., age, gender, education, handedness, pre-morbid representation of language, medical comorbidities, history of previous strokes, and regional atrophy in areas participating in recovery) and environmental contingencies (i.e., occupational and leisure activities and communication partners) [88]. Hence, it seems that individual differences on a number of factors accounts for the commonly observed heterogeneous patterns of spontaneous and therapy-induced recovery across aphasic individuals. Patients’ attitudes toward their aphasic syndromes may influence the recovery process as well. For example, patients with Broca’s aphasia are fully aware of their language deficits and, as a result, engage in more aphasia therapy than some Wernicke’s aphasic patients who, due to lack of insight of their language problems, are unwilling to participate in language training [89]. Therefore, the search for new and better factors predictive of PSA recovery is growing very rapidly. During the past decades, most experts have agreed that variability of language recovery after first-ever stroke is a frequent phenomenon and that the final outcome of aphasic deficits cannot be reliably predicted at 3 months post-onset by traditional demographic variables (e.g., age, sex, and handedness) and/or lesion characteristics (e.g., location and volume) [62, 90]. This is an important issue because a deeper understanding of these factors may improve the prediction of outcome in the acute stage and assist clinicians in the implementation of early therapeutic interventions [62, 90]. Some initiatives in this direction are emerging. Positron emission tomography and functional magnetic resonance imaging studies attempt to distinguish key regions of the brain that promote improvement of language deficits from others that may hinder full recovery through maladaptive reorganization [80, 82, 91, 92]. In complimentary terms, diffusion tensor imaging (i.e., white matter fiber tractography) reveals variability in the hemispheric lateralization of long white matter fiber pathways (e.g., arcuate fasciculus) [93] that may play a role in the clinical profile, prognosis, and recovery patterns of PSA [51]. Furthermore, tractography may also assist rehabilitation by identifying preserved structures participating in recovery as well as structural changes in white matter pathways triggered by intensive rehabilitation [94].
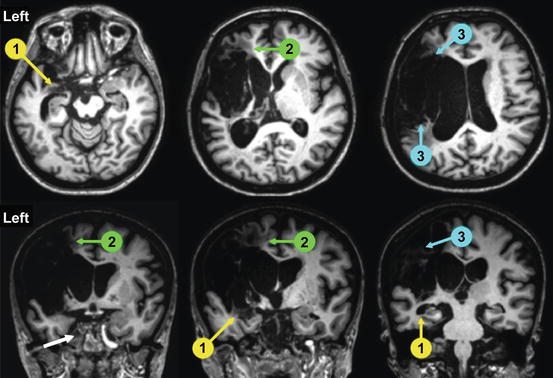

Fig. 6.4
Structural MRI of a patient with long-lasting (3 years) nonfluent aphasia (spontaneous speech limited to single words and perseverations) showing a large infarction in the left middle cerebral artery territory. Axial and coronal 3T MRI slices show the full extension of the lesion involving the fronto-temporoparietal cortex and extending deeply to insula, basal ganglia, and white matter tracts. Despite intensive aphasia therapy and a trial of memantine (20 mg/day during 3 months), no changes were noted in oral productive functions. Involvement of brain structures critical for recovery such as the hippocampus (1, yellow circles and arrows), subcallosum fasciculus (2, green circles and arrows), and arcuate fasciculus (2, blue circles and arrows) most likely accounted for the lack of recovery of verbal output
Treatment Interventions
Speech-Language Therapy
In light of what is known about spontaneous recovery from PSA [95], it can be expected that most aphasic patients will require therapeutic interventions to push recovery further. Aphasia rehabilitation begins during the acute hospitalization, as soon as patients regain ability to take part in language training. Although drugs can also be used in this phase (see later), speech-language therapy (SLT) remains the mainstay intervention for aphasia [1, 95]. Improvement of language deficits is highest in the initial 4 weeks after stroke [74], yet few studies of aphasia rehabilitation in the early post-stroke period have been performed. Several reasons can account for the paucity of rehabilitation trials in acute PSA. Recruitment of acute patients for early aphasia rehabilitation is not an easy enterprise, although a recent study demonstrated that randomized controlled intervention trials in acute PSA are feasible [96]. Reasons for exclusion or abandonment of SLT in acute PSA include medical complications (e.g., pneumonia and cardiac decompensation) or other factors inherent to the unstable neurological status including reduced alertness and attention span, aphasia severity, severe speech deficits (e.g., dysarthria, hypophonia, and apraxia of speech), lack of rehabilitation engagement due to fatigability, depression, apathy, aging (e.g., unwillingness to participate), or pre- and post-stroke dementia. Despite these disadvantages, recent studies in acute PSA have shown that 2 h/week of SLT is effective, but intensive interventions (i.e., 4 h/week) provide similar outcomes than less intensive regimes (i.e., 2 h/week), and most acute patients do not tolerate demanding regimes (i.e., 4 h/week) [89]. Another recent randomized controlled pilot study revealed that very early PSA therapy (range: 0–10 days after stroke onset) improved aphasia severity and communication deficits [97], but further studies are needed. The benefits provided by different aphasia therapies in patients with chronic PSA are undisputed, particularly when scientifically based interventions (e.g., constraint-induced aphasia therapy, errorless learning, and computer-based aphasia therapy) are implemented [59, 65, 98–100]. Most studies carried out in chronic PSA have demonstrated that aphasia therapy is efficacious when it is administered in an intensive and prolonged way. The fact that language deficits associated with PSA can be attenuated several years after onset demonstrates that the temporal window for aphasia recovery is much wider than previously estimated [59]. This new insight militates against the argument claiming that further benefits with aphasia rehabilitation cannot be expected 1 year after stroke onset. Figure 6.5 shows a diagram that will help clinicians and other health professionals (e.g., speech pathologists and neuropsychologists) to determine appropriate therapy options and timing for implementing different treatment strategies.