Fig. 20.1
Leak demonstrated on UGI 2 weeks after laparoscopic sleeve gastrectomy
Of note, many practices obtain routine UGI as part of postoperative algorithm for management, whether the patient is or is not exhibiting any specific signs of leak. Routine contrast studies, however, have low sensitivity for leaks, and prior work has shown alarmingly inconsistent detection rates for a leak, ranging from 50 to 79 % [20–22]. One important factor to consider is that the jejunojejunal anastomosis is difficult to assess by UGI, and although the risk of leak at this anastomosis is very low, it is still a possibility.
Treatment
Late leaks generally have a very different etiology and treatment strategy than early leaks, with the exception of late leaks that are associated with abscess formation, because this presentation may represent a contained early leak but with a delayed clinical presentation. In this case, immediate operation is not recommended due to prohibitive inflammation of the tissues and associated adhesions. Our patient underwent a CT of the abdomen and pelvis demonstrating a contained, 5 cm fluid collection anterior to the gastrojejunostomy.
Intraoperative Management
According to the literature, once a leak is suspected by clinical signs and symptoms, and then confirmed with CT, the rate of therapeutic laparotomy is 100 % [4]. Although in this scenario, the risk of false negative for abdominal exploration is virtually zero, other management approaches should be considered. Reoperation after bariatric procedures is associated with substantial potential morbidity and mortality. A nonoperative approach may be justified in certain patients. Patients with controlled leaks who are hemodynamically stable are candidates for nonoperative management. The nonoperative approach may include placement of a percutaneous drain, manipulation of indwelling surgical drains, percutaneous placement of a tube gastrostomy in the remnant stomach, NPO status, intravenous antibiotics, and gut rest/nutritional support via TPN. Innovative strategies with endoscopic drainage and endoscopic therapies may offer safe and efficacious alternatives to laparoscopic or open abdominal exploration (Fig. 20.2).
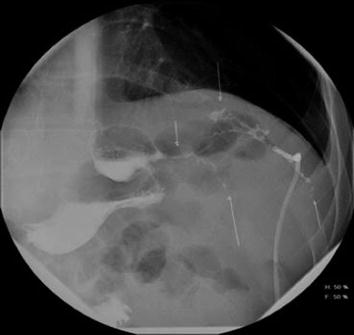

Fig. 20.2
Strictured vertical sleeve gastrectomy with leak and percutaneously placed drain
If operative management is necessary, several surgical principles are important to remember. Because of the many possible scenarios one might encounter intraoperatively, the surgeon should be prepared for diagnostic and technical challenges, and good surgical judgement is essential. A patient with a perforated marginal ulcer is managed ideally by closure of the edges of the perforated tissue and bolstered with omental tissue. If the ulcer edges are not amenable to the primary closure, an omental patch is the best option. Another option is the placement of a form of gastrostomy tube (use a Malecot) into the perforation and closure or patching of the area. For some patients with a history of marginal ulcers and stricture at the gastrojejunostomy, a complete revisional bariatric procedure of the gastrojejunal complex may be indicated in the hemodynamically stable, non-septic patient and when there is no local sepsis present. In our clinical scenario, the CT suggests a perforation at the gastrojejunostomy; however, the entire Roux-en-Y anatomy should be examined for factors that could contribute to the development of a marginal ulcer, such as a short Roux limb leading to the bile reflux or a breakdown in the gastric staple line closing the proximal pouch with a gastrogastric fistula to the remnant stomach. If the Roux limb is too short, then lengthening of the Roux limb with revision of the jejunojejunostomy is indicated. If there is a gastrogastric fistula, then closure of the fistula is imperative with placement of autogenous tissue between the staple lines.
One challenging scenario occurs when the site of perforation has sealed, so that on abdominal exploration, the exact site of perforation is not evident, although surrounding inflammation and fluid collection may be present. In this case, careful yet provocative maneuvers should be performed to identify the site of perforation, such as a leak test by insufflation under saline immersion. Uncovering of suspected areas by removal of adhered tissues and even gentle probing may be necessary to identify the site of leak. If no definitive leak can be identified at the gastrojejunostomy, other etiologies should be considered, particularly a duodenal or gastric perforation from indolent peptic ulcer disease or a leak at the staple line closure of the proximal pouch. If no other site of leak is identified, omental covering and wide drainage of the most likely site is recommended. When there is strong evidence of a healed marginal ulcer, some consideration should be entertained for revision of the size of the proximal pouch with reconstruction of the gastrojejunostomy.
After repair of the site of perforation, anastomotic revision, or downsizing of too large a proximal pouch, the authors advocate performing an intraoperative leak test by air insufflation using endoscopy or other means with submersion of the anastomosis under normal saline. If air bubbles are seen, the bubble stream is followed to the point of leak and the area is oversewn, as the condition of the tissues allow. The authors have used fibrin glue in the past but have abandoned this adjunctive measure due to concern that a heavy layer of fibrin glue may prevent adherence of omentum to the serosa; rather, a vascularized omental patch is utilized with tacking sutures.
An important adjunct to the treatment of anastomotic leak or perforation is the placement of a gastrostomy tube in the gastric remnant. This tube provides the ability to maintain a favorable nutritional status using enteric supplementation or full nutritional support to maximize healing. In obese patients, it is often impossible to use a proper Stamm technique because the stomach may not reach the abdominal wall due to immobility of the stomach and prominent visceral obesity. In these cases, a double purse-string suture is used to secure the tube at the stomach, and omentum can be wrapped around the tube in its course from the stomach to the abdominal wall; this technique will quickly provide a tissue tract and is safe and effective.
Practice is evolving in terms of a laparoscopic or open approach to abdominal exploration. For surgeons with advanced laparoscopic skills the authors suggest beginning with a laparoscopic exploration, but the operative approach should depend on the operating surgeon and their experience managing leaks with a laparoscopic intervention. The same principles of leak management apply. One consideration is that the laparoscopic exploration allows better visualization of the gastroesophageal junction, especially in the setting of morbid obesity. Additionally, laparoscopy lessens the risk of wound-related morbidity and slow healing time for larger incisions should they get infected, which can be the major problems in these patients. In contrast, the conventional benefits of laparoscopic surgery, including shorter stay, less pain, and quicker recovery, may not apply in this setting.
When the leak is identified and repaired, the authors suggest wide abdominal drainage using a drain which transverse the abdominal wall in a nondependent region to minimize drainage from around the tube. The authors prefer 19 F, channel drains placed with the tip in the left subdiaphragmatic space, coursing between the gastric remnant and the gastrojejunostomy, posterior to the Roux limb, and exiting the abdomen anteriorly in the right upper quadrant. Additional drains may be necessary along the medial aspect of the anastomosis depending on the extent of local sepsis and suppuration.
Enteric drainage via a nasogastric tube placed across the gastrojejunal anastomosis protects the repair from excessive intraluminal pressure. This drain can be guided intraoperatively under direct vision. Alternatively, an endoscopic technique is used most frequently by the authors. This technique is designed to prevent iatrogenic disruption of the repair: A flexible guidewire with a soft, flexible tip is passed from the mouth through anastomosis under direct vision with the endoscope; the endoscope is withdrawn leaving the wire in place across the anastomosis; then a soft rubber, non-sump enteric tube (such as “red Robinson” catheter) is advanced over the wire. Once the enteric tube is confirmed to be in optimal position across the anastomosis, the wire is removed, and the proximal end is passed from an oral to nasal location; this repositioning of the tube is accomplished by passing a traditional nasogastric tube from the nasal cavity out through the mouth where it is sutured to the soft rubber enteric tube and then threaded back through the nasal passage. The tube is then placed to gravity drainage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








