History includes agent, time of ingestion, approximate amount of ingestion, route of exposure, and resulting symptoms. All sources of information should be sought (observers at the scene, EMS personnel, current and old prescription bottles from the scene, medical alert bracelets, prescriptions, hospital and clinic records, etc.). Patients with nonaccidental overdose may fail to reveal all of the agents ingested.
Physical examination may be helpful in suggesting a particular agent or toxidrome. Blood pressure, temperature, pulse, respiratory pattern, skin (presence of needle marks, bullae, cyanosis, erythema, dermatographism, etc.), diaphoresis, respiratory and cardiovascular status, bowel sounds, the odor of the breath, and neurologic function should all be assessed. The neurologic examination should specifically assess mental status, pupillary size, focal deficits, deep tendon reflexes, the presence of nystagmus, and the gag reflex.
Routine laboratory assessment in overdose patients generally includes pulse oximetry, a complete blood count (CBC), electrolytes, BUN, creatinine, blood sugar, anion gap, urinalysis, and electrocardiogram (ECG). History and clinical status guide further studies such as specific drug levels or toxicology screens.
The regional Poison Control Center can assist in identifying particular agents, predict the anticipated toxic effects or severity of exposure, recommend further diagnostic evaluation, and help guide therapy. In most seriously ill patients, general and specific therapeutic measures must be undertaken before toxicologic confirmation of a particular substance. Toxicology screens vary by laboratory and detect limited types of ingestants.
Qualitative determination of a substance may be sufficient to guide therapy. However, quantitative levels of some substances are required to treat specific overdoses (Table 64-1).
Skin decontamination should be performed when percutaneous absorption of a substance may result in systemic toxicity or when the contaminating substance may produce local toxic effects.
Table 64-1 Intoxications in which Quantative Levels are Helpful
Acetaminophen
Arsenic
Carbamazepine
Carboxyhemoglobin
Depakote
Digoxin
Ethanol
Ethylene glycol
Iron
Lead
Lithium
Mercury
Methanol
Methemoglobin
Phenobarbital
Phenytoin
Salicylates
Theophylline
Valproic acid
Decontamination is necessary for corrosives, hydrocarbons, metals, salicylates, pesticides, cyanide, methanol, irritants (e.g., Mace), and radioactive isotopes (see Chapter 53).
Treating staff should take appropriate measures to prevent exposure, as directed by the agent. The patient’s clothing should be removed and sealed in plastic bags. Involved surfaces of the skin should be washed at least twice with a mild soap and water. Particular attention should be paid to body folds, nails, and hair when appropriate.
Gastrointestinal decontamination
Emesis. There is little evidence that the use of emetics (syrup of ipecac) improves the outcome of patients with toxic overdoses. In addition, the use of emetics can lead to adverse outcomes, particularly aspiration pneumonia. Hospital use of syrup of ipecac is not recommended.
Gastric lavage. If the patient presents within 1 to 2 hours of ingesting a significant toxic substance and is symptomatic (obtunded, comatose), orogastric lavage after endotracheal intubation, followed by activated charcoal with sorbitol added as a cathartic, is recommended. For an asymptomatic patient, emesis, lavage, catharsis, or activated charcoal is not recommended unless the patient is seen within 1 to 2 hours and there is evidence of the ingestion of large amounts of a toxic drug, in which situation activated charcoal is recommended.
Contraindications for gastric lavage include the ingestion of caustics when perforation of the esophagus may have occurred or may be caused, the ingestion of hydrocarbons of low viscosity when aspiration may be caused, and the ingestion of glass or other sharp material.
Small-diameter nasogastric tubes are not effective. Number 28- to 40-French Ewald tubes should be used in adults and number 16- to 26-French orogastric
tubes used in children. After placement, the position of the tube is confirmed by passing air into the tube with a 50-mL syringe and listening over the stomach for “bubbling.” If any doubt exists as to tube location and gastric contents cannot be aspirated, the position of the tube should be confirmed by x-ray before lavage.
For lavage, the patient is placed in the left lateral decubitus position, the stomach emptied as completely as possible by aspiration, and normal saline or tap water instilled into and then suctioned out of the stomach. Aliquots should be 200 to 300 mL in adults and 50 to 100 mL in children. Lavage is continued until the return is clear or, if the initial return is clear, after 2 L of solution has been used. The first 100 mL of lavage solution or gastric aspirate should be separately collected for toxicologic analysis.
Activated charcoal should be considered unless specifically contraindicated. Activated charcoal is relatively contraindicated when an oral antidote or endoscopy will be used (e.g., ingestions of caustics). In adults, the dose of activated charcoal is 1 g/kg with a minimum dose of 30 g; the dose in children is 1 g/kg. The method of administration is to dilute the appropriate amount of activated charcoal in approximately four parts of water or sorbitol. The slurry is then instilled by the lavage tube or given to the awake patient orally. Patients with vomiting should be controlled with ondansetron prior to attempting charcoal administration. Activated charcoal is tasteless but has an unpleasant appearance and gritty texture. Toxic ingestions with drugs having an enterohepatic circulation, such as theophyllines, phenobarbital, the tricyclic antidepressants (TCAs), the phenothiazines, and digitalis, generally require that activated charcoal be readministered every 4 to 6 hours to limit reabsorption during recirculation. The administration of multiple doses of charcoal has also been reported to be effective in dapsone, digitoxin, salicylates, phenytoin, propoxyphene, carbamazepine, phenylbutazone, nadolol, and meprobamate overdoses. Activated charcoal does not adsorb simple alcohols such as ethanol or methanol; strong acids or bases; or simple ions such as iron, lithium, or cyanide (Table 64-2).
Cathartics are contraindicated in infants, in patients receiving an oral antidote, and in patients with adynamic ileus, severe diarrhea, intestinal obstruction, abdominal trauma, or recent abdominal surgery. Magnesium-containing cathartics are contraindicated with renal failure or when the ingested substance is likely to be associated with renal injury. Sodium sulfate should not be given to patients with hypertension, significant left ventricular dysfunction, or congestive heart failure. Laxatives should not be used for catharsis, because activated charcoal may bind these agents and render them ineffective. Adults may be given magnesium citrate, sorbitol (150 g maximum), or sodium phosphate, 15 to 30 mL diluted 1:4 with water. Children may be given magnesium citrate, 4 mL/kg. Sorbitol is also probably safe in children; however, sodium phosphate should not be given because of the probability of severe electrolyte imbalances.
Enhanced elimination. Acceleration of elimination by diuresis, dialysis, or hemoperfusion may be useful therapeutic options in specific ingestions. An important measure of a particular drug’s ability to be actively eliminated is its volume of distribution (Vd), which is conceptualized as the hypothetical volume of body water that would be required to contain the amount of drug ingested at the same concentration as that found in the blood. Vd is expressed in liter per kilogram and is defined for each particular substance. In general, Vd is a measure of whether a drug is highly
tissue soluble (large Vd) or remains confined within the vascular compartment (small Vd). A substance with a large Vd, therefore widely distributed within tissues, is not very amenable to diuresis, dialysis, or hemoperfusion. Conversely, a substance with a small Vd, therefore relatively confined within the vascular space, may be amenable to enhanced elimination by one of these techniques.
Table 64-2 Activated Charcoal Absorption of Commonly Encountered Drugs and Toxins
Well absorbed
Amphetamines
Antidepressants
Antiepileptics
Antihistamines
Atropine
Barbiturates
β-Blockers
Cimetidine
Digitalis preparations
Ergot alkaloids
Furosemide
Glutethimide
Indomethacin
Meprobamate
Opioids
Phenothiazines
Phenylbutazone
Phenylpropanolamine
Quinidine
Strychnine
Tetracycline
Theophylline
Moderately absorbed
Acetaminophen
Benzene
DDT
Disopyramide
Kerosene
Malathion
Mexiletine
Nonsteroidal anti-inflammatory drugs
Phenol
Tolbutamide/chlorpropamide
Poorly absorbed
Acids, strong
Alkalis, strong
Cyanide
Ethanol
Ethylene glycol
Iron
Lithium
Methanol
Diuresis. Forced diuresis may enhance elimination of a drug if excretion is primarily renal and if the substance is polar and has a relatively small Vd with little protein binding. Forced diuresis is accomplished by establishing a urinary flow rate of 5 to 7 mL/kg/h using IV supplemental solute, such as 0.45% normal saline and spaced boluses of furosemide (20 mg). Forced diuresis is contraindicated in patients with hypotension and pulmonary edema. Serum electrolytes must be monitored because both hyponatremia and hypokalemia may develop; in addition, mannitol may induce hyperosmolality. The total dose of mannitol, if this agent is chosen, should never exceed 300 g, and in most patients, 20 to 100 g is sufficient.
Alkaline diuresis. Alkalinization of the urine promotes the ionization of weak acids, thereby preventing their reabsorption by the kidney and thus facilitating elimination. Alkaline diuresis is useful for toxic amounts of the long-acting barbiturates (phenobarbital, mephobarbital, primidone), salicylates, lithium, and isoniazid.
Alkaline diuresis is accomplished by giving adults 1 ampule of sodium bicarbonate (NaHCO3) IV, followed by a constant infusion of 1 to 2 ampules of NaHCO3 in 1 L of 0.25% to 0.45% normal saline to maintain the urine at the desired pH of approximately 7.3 to 8.5. Furosemide should be given concomitantly, along with supplemental solute such as 0.45% normal saline, to increase urine output to approximately 5 to 7 mL/kg/h. Complications of alkaline diuresis include metabolic alkalosis, hypernatremia, hyperosmolality, and fluid retention. In salicylate intoxication, which frequently is accompanied by dehydration, the patient must be rehydrated before the institution of forced alkaline diuresis, and dextrose should be included in the infusion.
Acid diuresis. Acidification of the urine may enhance the elimination of weak bases by way of the mechanism noted in “Gastric Lavage” discussed under the section “General Assessment and Management.” Substances optimally excreted should have a small Vd, little protein binding, and be excreted primarily by the kidney.
Dialysis. Hemodialysis has an important but limited role in the active elimination of intoxicating substances. The ability of a particular substance to be dialyzed depends on a number of factors including its molecular weight, lipid solubility, Vd, extent of plasma protein binding, and the ability of a concentration gradient to be maintained in the dialysate.
Immediate dialysis is indicated for patients ingesting toxic amounts of ethylene glycol, methanol, and paraquat. When other substances amenable to dialysis are involved (e.g., theophylline, lithium, the salicylates, the long-acting barbiturates, bromide, and ethanol), indications for dialysis include severe intoxication with progressive deterioration despite intensive supportive therapy or significant impairment of excretory function.
Hemoperfusion is a technique in which anticoagulated blood is passed through a column containing activated charcoal or resin particles. Although many of the same physical limitations of hemodialysis apply to the ability of a substance to be successfully removed by hemoperfusion, such factors as lipid solubility and molecular weight are circumvented.
Hemoperfusion should be considered for severe intoxications unresponsive to intensive supportive care with barbiturates, glutethimide, methaqualone, ethchlorvynol, meprobamate, chloral hydrate, and certain other agents. Complications of hemoperfusion include platelet depletion; reduction of plasma calcium, glucose, and fibrinogen; and transient leukopenia. Hemoperfusion cannot correct electrolyte abnormalities.
Exchange transfusions. Exchange blood transfusions are used principally in the treatment of severe poisonings involving hemolysins and methemoglobinemia.
Immunotherapy. The recent introduction of digoxin-specific antibody fragments has proved successful in treating patients with serious digoxin toxicity. Hyperimmune antisera are available for the treatment of certain snake and arthropod envenomations.
Antidotes are important with specific ingestions. Very few substances have specific antidotes. Antidotes reverse the physiologic effects of specific substances through a variety of differing mechanisms. Although some antidotes are nontoxic, others can cause severe toxic effects if used improperly. Table 64-3 lists a variety of substances and their specific antidotes. Details regarding the use of each antidote are further discussed under the management of specific ingestions.
Table 64-3 Antidotes Used in the Emergency Department | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
of the agent, intravenously if required, to terminate seizures, followed by its gradual withdrawal; phenobarbital may alternatively be used.
Clinical presentation. Central nervous system (CNS) and respiratory depression (including coma and respiratory failure), noncardiogenic pulmonary edema, hypothermia, miosis, bradycardia, hypotension, and decreased GI tract motility are characteristic narcotic toxicity. These findings occur within minutes of an IV dose and within 20 to 30 minutes of oral ingestion. Typically, the respiratory depressant effects are longer lived than the analgesic effects of many of the narcotics and specific respiratory effects. Meiosis may be absent with meperidine overdose and may be absent hypoxemia. Death is due to respiratory depression.
Laboratory studies should be guided by clinical status. Toxicology specimens may be sent for qualitative analysis.
Pentazocine, propoxyphene, or Lomotil may present differently from other narcotics. Pentazocine is more likely to cause dysphoria, delusions, and hallucinations. Hypertension, tachycardia, flushing, chills, and diaphoresis are seen. Propoxyphene frequently causes seizures and cardiac rhythm dysrhythmias. Concomitant ethanol ingestion may greatly increase toxicity. Death is due to cardiac and respiratory arrest. Lomotil intoxication, especially in children, is characteristically biphasic. The first phase, due to anticholinergic effects of atropine, is marked by hyperpyrexia, flushing, hallucinations, lethargy, urinary retention, and tachycardia. After 3 hours, the characteristic findings of narcotic intoxication dominate the clinical picture.
Treatment of narcotic overdose includes IV administration of naloxone, a specific opiate antagonist. There are no contraindications. A period of close in-hospital observation of respiratory status is also indicated. The IV administration of naloxone rapidly restores CNS and cardiopulmonary function in patients intoxicated with a narcotic agent; onset of action is 1 to 2 minutes when given intravenously with an effect lasting up to 60 to 90 minutes. Naloxone may be given subcutaneously, intramuscularly, sublingually, or by endotracheal tube if venous access cannot be established. In adults, the recommended dose is 0.8 to 2.0 mg every 5 minutes up to 10 mg, and 0.01 to 0.1 mg/kg in children. Patients ingesting toxic amounts of propoxyphene may initially require extremely large doses of naloxone. Importantly, when only a partial response to naloxone occurs, other coexisting factors such as a mixed overdose, hypoglycemia, head trauma, Wernicke encephalopathy, hypoxia, or posthypoxic encephalopathy should be considered.
Because the half-life of many narcotic agents is significantly longer than that of naloxone (T1/2 = approximately 1 hour after IV or endotracheal tube administration), respiratory or cardiac depression may recur as blood levels of naloxone fall. Because most deaths in patients with narcotic intoxication are related to respiratory arrest, efforts to ensure adequate respiratory function are paramount. With severe ingestions or those associated with long-acting agents, a continuous IV naloxone
infusion is required. Two mg of naloxone in 500 mL of normal saline is administered at 100 to 200 mL/h or titrated to the desired clinical response. Noncardiogenic pulmonary edema may occur in patients with toxic amounts of narcotics and is managed with oxygen, positive pressure ventilation if required, and naloxone administration. Routine measures undertaken in patients with cardiogenic pulmonary edema are of no benefit. Seizures may occur and are treated with IV diazepam and phenytoin. Cardiac arrhythmias should be treated with an appropriate agent.
Lethargic or comatose patients require endotracheal intubation. Patients with prolonged symptoms may be admitted for observation. Naloxone infusion may be indicated for 12 to 48 hours, depending on the agent involved. Diuresis, dialysis, and hemoperfusion are not indicated.
Symptoms and signs of heroin withdrawal are manifest after a latency period of approximately 12 hours; lacrimation, restlessness, rhinorrhea, “gooseflesh,” insomnia, diaphoresis, muscle cramps, pupillary dilation, hot and cold flashes, vomiting, diarrhea, and fever are noted. Symptoms usually begin to subside after 72 to 96 hours of abstinence. Although most hospitals do not primarily manage detoxification from narcotics, admissions related to concomitant acute medical or surgical illness may be necessary. One approach is to administer methadone, 10 mg orally or intramuscularly when signs of opiate withdrawal appear, and to readminister additional 10-mg doses when objective signs of opiate withdrawal are noted. Further doses may be required every 8 to 12 hours; doses should be reduced approximately 50% every 2 days until detoxification is complete. Importantly, patient estimates of daily narcotic dose are generally inaccurate; the use of this estimate to calculate a starting dose of methadone is not appropriate.
Clinical presentation. Early symptoms of barbiturate intoxication include drowsiness, slurred speech, or paradoxic excitement. More severe intoxication results in increasing sedation and progressive coma. Doses approximately three-times the hypnotic dose decrease the respiratory drive. Physical findings include initially reactive meiosis or, if CNS hypoxia secondary to respiratory depression has occurred, mydriasis. Respiratory depression may progress to apnea. Hypothermia and decreased GI motility are characteristic. Chronic users and acute overdoses show bullous skin lesions over pressure points on the hands and knees due to pressure stasis. Hypotension develops due to increases in venous capacitance and the direct myocardial depressant effects. Profound CNS depression associated with a flatline electroencephalogram (EEG) may be seen in severely intoxicated patients.
Laboratory data may reveal acidosis and hypoxia. Qualitative identification of the specific barbiturate is much more important than quantitative levels, because
the clinical assessment of the severity of intoxication guides management and the phenomenon of tolerance is well recognized in patients chronically ingesting barbiturates.
Treatment consists of intensive supportive therapy. Lavage should be performed up to 8 hours after ingestion because GI emptying is typically delayed; phenobarbital may form concretions necessitating endoscopic removal. Hypothermia is managed with the usual techniques. Hypotension is treated initially with fluid resuscitation followed by pressors. The long-acting drugs demonstrate an increased rate of elimination when alkaline diuresis is used; the urinary pH should be adjusted to between 7.5 and 8.0 with intravenously administered NaHCO3. The details of alkalinization are discussed in “Gastric Lavage” discussed under the section “General Assessment and Management.” Severe barbiturate intoxication that does not respond to intensive conservative therapy may respond to hemodialysis or hemoperfusion, particularly long-acting agents with hepatic or renal failure.
Barbiturate withdrawal is manifest by insomnia, excitement, delirium, hallucinations, toxic psychoses, tremors (which may be dramatic), nausea, vomiting, orthostatic hypotension, and seizures. Treatment consists of phenobarbital, followed by its gradual withdrawal.
Clinical presentation. Amphetamine intoxication ranges from restlessness and irritability to seizures and coma. Insomnia, tremor, diaphoresis, mydriasis, confusion, tachypnea, nausea, vomiting, nystagmus, tachycardia, hyperpyrexia, delirium, marked hypertension, and arrhythmias may occur. Amphetamine toxicity includes toxic psychoses, repetitive behaviors, renal failure, coagulopathy (caused by hyperthermia), and intracranial hemorrhage secondary to severe or accelerated hypertension. Because tolerance to the amphetamines occurs, quantitative serum levels do not reliably predict toxicity.
The treatment of amphetamine toxicity is supportive; GI tract decontamination is undertaken if indicated, and seizures are treated with IV diazepam. Psychotic and behavioral disturbances should not be treated with chlorpromazine or haloperidol because these agents may potentiate seizures. Lorazepam 2 to 3 mg IV in an adult may control agitation. Hyperthermia must be aggressively treated with cooling blankets, and hypertensive crises may be treated with an alpha-blocking agent or nitroprusside. Beta-blockers alone should not be used to treat hypertension caused by amphetamines because they may raise the blood pressure due to unopposed alpha effects. Labetalol may be considered because it has alpha- and beta-adrenergic blocking effects.
Excretion of amphetamines is enhanced with acid diuresis using mannitol or furosemide and ammonium chloride (1-2 g orally or IV every 4-6 h) to achieve urinary flow rates of 5 to 7 mL/kg/h and a urine pH of less than 5.5. Acid diuresis is contraindicated in the presence of seizures and serious hyperthermia, because
myoglobinuric renal failure may ensue. Both hemodialysis and hemoperfusion are effective in removing amphetamines but are not required except for progressive or prolonged toxicity despite supportive therapy and forced diuresis.
The clinical presentation is similar to amphetamine intoxication: CNS stimulation, tachycardia, chest pain, hypertension, hyperthermia, muscle twitching, seizures, nausea, vomiting and ventricular dysrhythmias, followed by CNS, cardiac, and respiratory depression. Patients may also have rhabdomyolysis and myoglobinuria. Death usually results from respiratory failure or cardiac infarction and arrest.
Treatment is supportive; GI decontamination is not indicated. Ativan or diazepam should be used to control seizures, followed by phenobarbital if needed. Beta-blockers, when used alone, may cause an unopposed alpha-adrenergic reaction with further elevation of the blood pressure; therefore, phentolamine with esmolol can be used, or labetalol alone can be used, which itself has both alpha- and beta-adrenergic blocking activity. Hypotension in the depressive phases of intoxication should be treated with fluid expansion and vasopressors such as dopamine. Hyperthermia should be controlled with cooling blankets. Cocaine-induced psychosis has been successfully treated with neuroleptics. Forced diuresis, dialysis, and hemoperfusion are ineffective.
Clinical features of strychnine toxicity include fever, muscle stiffness, hyperreactive reflexes, tetanic convulsions, opisthotonus, rhabdomyolysis, lactic acidosis, and death caused by respiratory failure.
Treatment is supportive; airway control should be obtained and the gastric lavage initiated if within 1 hour of ingestion. Charcoal may bind some of the agents. Seizures are treated with diazepam, and a nonstimulating environment should be provided. Forced diuresis and dialysis are of little value because of the rapid urinary excretion of the agent.
Clinical findings include weakness, nausea, vomiting, abdominal pain, blurred vision, mydriasis, tachycardia, hyperpyrexia, and hyperreflexia. Visual hallucinations and perceptive distortions are also common.
Treatment is supportive. The patient should be reassured as much as possible. If sedation is required, a benzodiazepine should be considered. Phenothiazines should be avoided (see below).
Clinical presentation is characterized by a variety of sympathomimetic effects, euphoria, and psychic effects similar to mescaline and the tryptamines at high doses. MDA has been associated with coma, seizures, and cardiovascular collapse when ingested in high doses.
Treatment involves reassurance and conservative support as well as respiratory and circulatory assistance as needed. The patient should be placed in a monitored bed. The use of phenothiazines should be avoided because they lower the seizure threshold. Seizures should be treated with diazepam.
Distortions of time, space, and somatic sensation mark the clinical presentation; delusions, hostility, and bizarre and violent behavior are frequent. Vertical, rotatory, and horizontal nystagmus occurs, the pupil size is variable, and the corneal reflex may be decreased. Ataxia, dystonic posturing, muscle twitches, seizures, rhabdomyolysis, myoglobinuria, hyperthermia, and mild hypertension may also be noted. Respiratory stimulation is followed by depression and apnea in patients with severe intoxications.
Laboratory data frequently demonstrate hypoglycemia and mild elevation of creatine kinase, AST, ALT, and myoglobin.
The treatment of PCP intoxication should focus on reassuring the patient; diazepam or lorazepam may be used for sedation. Phenothiazines, such as haloperidol, should be used with caution because their use may result in hypotension and reduce the seizure threshold.
Activated charcoal should be administered initially and, because of enterohepatic recirculation, readministered at 4- to 6-hour intervals. Seizures are treated with IV diazepam and phenytoin if necessary.
Clinical presentation. During the initial 12 to 24 hours after ingestion (phase 1), signs and symptoms of severe acetaminophen overdose consist of mild nausea, vomiting, anorexia, diaphoresis, and pallor. Phase 2 is characterized by a relatively complete return to well being lasting as long as 4 days. During this period, laboratory data demonstrate a rising AST and other hepatic enzymes; mild right upper quadrant abdominal pain may be noted. In phase 3, which typically develops 3 to 5 days after ingestion in untreated patients, patients become symptomatic from hepatic injury. Symptoms include anorexia, nausea, malaise, abdominal pain, progressive evidence of hepatic failure, coma, and death. In those who survive, liver function tests return to normal within a few weeks; serious toxicity and death are rare in children for reasons that are not clear.
Laboratory studies. The level of plasma acetaminophen 4 hours or more after the ingestion is critical; this test serves to predict the extent of potential toxicity and to guide specific management. The Rumack-Matthews nomogram or the regional poison center will assist in the interpretation of blood levels. It is very important that all reported levels, as well as the reported units, be double-checked. For example, some laboratories will report acetaminophen levels in micrograms/deciliter rather than micrograms/milliliter. If this is not recognized, the physician will make a major error and underestimate the actual level. In general, patients with 4-, 6-, 8-, or 12-hour levels greater than 150, 110, 75, or 40 μg/mL, respectively, require specific treatment with N-acetylcysteine as outlined. Levels less than these parameters
are not expected to be toxic, and specific treatment with N-acetylcysteine is not indicated. Similarly, if a calculated dose can be relied on (which it often cannot), ingestions of less than 7 g in the adult or less than 100 mg/kg in children are not expected to produce toxicity and, in general, patients ingesting these amounts or less do not require specific treatment. It is important to note that some patients, particularly the elderly or alcoholic patient, may sustain injury from ingestion of less than 7 g (i.e., even with acceptable daily doses of acetaminophen). Such patients may present with unexplained liver dysfunction. In general, however, adults with calculated doses greater than 7 g or children ingesting more than 100 to 150 mg/kg require treatment and a 4-hour or later plasma acetaminophen determination to guide specific therapy. Plasma acetaminophen levels drawn after the institution of treatment with N-acetylcysteine are unreliable as to toxicity and should not be used. Baseline liver function tests should be drawn in all patients.
Gastrointestinal decontamination. Since acetaminophen is avidly bound by activated charcoal, the administration of this agent is recommended. Repeat doses are not recommended in acetaminophen ingestions alone, because absorption is rapid and a very limited enterohepatic circulation exists. Cathartic agents have a limited role in isolated acetaminophen ingestions.
Specific treatment with N-acetylcysteine. N-acetylcysteine (Mucomyst) is given orally as 140 mg/kg, immediately followed by 70 mg/kg every 4 hours for an additional 17 doses. Most commonly 20% Mucomyst, containing 200 mg/mL of N-acetylcysteine, is diluted 1:3 with fruit juice or a soft drink (to mask its unpleasant taste). Five milliliters of 20% undiluted Mucomyst contains 1 g of N-acetylcysteine. If the dose is vomited within 1 hour of administration, it should be repeated, and in some patients, a nasogastric tube may be required to deliver the proper dose. N-acetylcysteine is most effective if given within 8 to 16 hours of ingestion but may exert beneficial effects if instituted within 24 hours. N-acetylcysteine may also be administered intravenously. Contact a regional poison control center for guidance. Seger and Murray have prepared a useful algorithm for the treatment of patients with acetaminophen ingestions (see Fig. 64-1). Patients presenting very early (within 1 hour of ingestion) can be given sorbitol along with activated charcoal; a 4-hour postingestion level of acetaminophen should be obtained and specific treatment with N-acetylcysteine initiated if the patient’s level on the Rumack-Mathew nomogram (Fig. 64-2) exceeds that associated with hepatic toxicity. Patients presenting between 4 and 8 hours after ingestion should have an immediate acetaminophen level drawn; patients with levels exceeding those in the nomogram for hepatic toxicity should be treated with N-acetylcysteine. Patients presenting between 8 and 24 hours of ingestion should have an immediate acetaminophen level drawn and treatment with N-acetylcysteine initiated if the amount of ingestion is unknown or if the amount of acetaminophen ingested exceeds 140 mg/kg. Treatment can be discontinued if nontoxic levels are determined to be present. If the history is reliable, and the calculated amount of acetaminophen ingested is less than 140 mg/kg, treatment can be delayed pending the serum level; treatment with NAC should proceed based on levels exceeding those in the nomogram.
Patients presenting after 24 hours of ingestion require an acetaminophen level and liver function studies; the regional poison center should be consulted with these results, because delayed treatment with N-acetylcysteine may be appropriate in certain patients with acetaminophen detectable in the serum, particularly with laboratory evidence of hepatic injury. Consult the regional poison center when acetaminophen is ingested with other substances.
Although children younger than 6 years old, in comparison with adults, have an increased sulfation capacity in relation to acetaminophen metabolism and a high turnover rate of glutathione, and therefore may be less susceptible to acetaminophen toxicity, this has not been clearly demonstrated. Treatment of children is therefore similar to that of adults. Treatment of pregnant patients is identical to that outlined. N-acetylcysteine does not appear to result in fetal injury. Although there are no clear guidelines for the management of patients with multiple-dose acetaminophen ingestions occurring over a period of time (the nomogram is intended for single acute ingestions only), some centers recommend consideration of N-acetylcysteine therapy when the total dose of acetaminophen over a 24-hour period exceeds 150 mg/kg or when laboratory evidence of hepatic injury is present. The regional poison center should be consulted in these cases. See Table 64-4 for some special considerations.
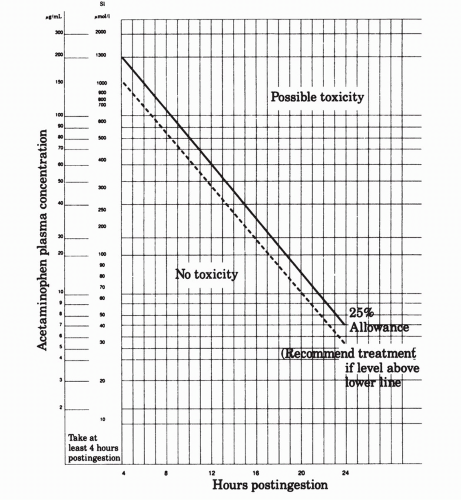 Figure 64-2. Acetaminophen toxicity. (From Rumack BH, Mathew H. Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871, with permission.) |
Acute toxicity. The absorption of acetylsalicylic acid (ASA) is rapid and complete after the oral ingestion of normal doses; ingestion of toxic amounts may delay gastric emptying, and enteric-coated preparations are absorbed erratically and to a variable degree. ASA is rapidly biotransformed to salicylic acid; this is further metabolized in the liver to salicyluric acid, acyl and phenolic glucuronides, and to gentisic acid. Salicylates administered in therapeutic doses for the treatment of a variety of inflammatory conditions (4-6 g/day) result in a half-life of approximately 6 to 12 hours; in lower doses, used for the routine suppression of fever, a half-life of 2 to 3 hours is noted. In toxic ingestions, as a result of the saturation of metabolic pathways, the effective serum half-life may increase dramatically to more than 20 hours. Importantly, in toxic ingestions, 50% or more may be excreted unchanged in the urine.
The pathophysiology of salicylate toxicity is complex. Salicylates directly stimulate the CNS (including the medullary respiratory center), resulting in the uncoupling of oxidative phosphorylation. Both the Krebs cycle dehydrogenase enzymes and amino acid metabolism are inhibited, which interferes with both platelet and vascular hemostatic mechanisms. These diverse effects combine to produce the various manifestations of toxicity.
CNS stimulation may result in hallucinations, confusion, irritability, anxiety, seizures, cerebral edema, and coma. Stimulation of the medullary respiratory center
produces hyperpnea and tachypnea, resulting in respiratory alkalosis. Respiratory alkalosis is compensated for by the exchange of intracellular hydrogen ions for extracellular cations and a renal bicarbonate diuresis. This contributes to metabolic acidosis and dehydration. The uncoupling of oxidative phosphorylation decreases the production of adenosine triphosphate, resulting in accelerated activity of the glycolytic and lipolytic pathways. Increased glycolysis and lipid metabolism result in production of lactic and pyruvic acids and acetone and acetoacetate, respectively. These organic acids also contribute to the development of metabolic acidosis, which is characterized by a high anion gap; their presence at the kidney, as an additional solute load, further enhances fluid loss. Furthermore, inhibition of amino acid metabolism results in an increase in serum amino acids, oxaloacetate, and α-ketoglutarate. These organic acids contribute further to the solute diuresis and the evolving anion gap. Dehydration is also caused by CNS-induced hyperventilation and from emesis, the latter resulting from the local irritant effects of the salicylates. Increased metabolic activity, particularly in children, may result in hyperthermia.
Table 64-4 Special Treatment Considerations for Acetaminophen (APAP)
Situation
Treatment
Vomiting after N-acetylcysteine (NAC) administration
Administer ondansetron 30 min before repeat administration of NAC.
APAP overdose in pregnant woman
Consult toxicologist. Consider IV NAC.
APAP overdose in chronic alcoholic
Treat with NAC at levels below nomogram treatment line.
APAP overdose in child
Use nomogram to determine treatment.
Chronic APAP ingestion
Treat with NAC if elevated liver function tests or any APAP present in serum.
Charcoal administration after overdose
Do not need to increase NAC dose
IV, intravenous.
From Seger D, Murray L. Aspirin, acetaminophen, and nonsteroidal agents. In: Rosen P, et al., eds. Emergency medicine, concepts and clinical practice, 4th ed. St. Louis: CV Mosby; 1998:1258, with permission.
Glucose metabolism is uniformly deranged, but individual variability in blood sugar levels is common. Diabetics, for example, may present with and maintain severe hypoglycemia; the reasons for this are not clear. In other patients, the blood sugar is initially somewhat elevated, presumably as a result of increased mobilization and metabolism of lipids and hepatic glycogen; however, as toxicity increases or persists, blood sugar may fall. The impairment in glucose metabolism may also be manifest by delayed hyperglycemia; in all patients, blood glucose levels must be followed closely. It should be noted that CNS glucose concentrations might be decreased even with a normal plasma glucose concentration.
Disturbed hemostasis is common and results from decreases in prothrombin and factor VII production, decreased platelet adhesiveness, and increased capillary fragility and permeability. The latter may be the cause of pulmonary edema seen in some patients as well as a contributor to the facial petechiae and subconjunctival hemorrhages seen in others.
Clinical presentation. Symptoms and physical findings include nausea, vomiting, abdominal and chest pain, tinnitus, hyperventilation, diaphoresis, hyperpyrexia, mental status abnormalities ranging from irritability and anxiety to coma, variable degrees of dehydration and oliguria, pulmonary edema, and signs of deficient hemostasis.
Children are much more likely than adults to have hyperpyrexia, early acidosis, and CNS symptoms, whereas older children and adults usually present with alkalosis during the initial period of acute salicylate intoxication.
Laboratory data should include a CBC, electrolytes, blood sugar, BUN, creatinine, arterial blood gases, liver function studies, prothrombin time, and partial thromboplastin time. A chest roentgenogram and ECG should also be obtained. Toxicology specimens should include salicylate levels on presentation and 6 hours after ingestion; additional levels drawn at 8 to 10 hours postingestion may be helpful to assess delayed salicylate absorption and elimination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






