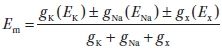Neurons maintain a membrane potential that is mainly influenced by the concentration difference of potassium (K) and sodium (Na) inside and outside of the neuron and the conductance of the membrane to these ions. At rest, the cell has much greater permeability to K and a resting potential around −60 mV; with depolarization, the conductance to Na is increased and action potentials are generated.
 Neurons communicate with each other across a synapse by releasing neurotransmitters that activate receptors on the postsynaptic neurons and change the conductance of these neurons to ions. Glutamate, an excitatory transmitter, increases the conductance to Na, K, and calcium (Ca); this leads to depolarization and increased excitability. Gamma-aminobutyric acid (GABA), an inhibitory transmitter, increases the conductance to chloride (Cl); this leads to a hyperpolarization and decreased excitability. Anesthetics reduce excitability by enhancing GABA inhibitory activity and reducing glutamate excitatory activity.
Neurons communicate with each other across a synapse by releasing neurotransmitters that activate receptors on the postsynaptic neurons and change the conductance of these neurons to ions. Glutamate, an excitatory transmitter, increases the conductance to Na, K, and calcium (Ca); this leads to depolarization and increased excitability. Gamma-aminobutyric acid (GABA), an inhibitory transmitter, increases the conductance to chloride (Cl); this leads to a hyperpolarization and decreased excitability. Anesthetics reduce excitability by enhancing GABA inhibitory activity and reducing glutamate excitatory activity.
 The brain and spinal cord need adenosine-5′-triphosphate (ATP) for energy-requiring processes such as ion pumps; transporting molecules, amino acids, and proteins; and synthesizing proteins and lipids. Most ATP formation comes from the metabolism of glucose in the presence of oxygen; when oxygen is limited, ATP production from glucose is much less and lactic acidosis occurs.
The brain and spinal cord need adenosine-5′-triphosphate (ATP) for energy-requiring processes such as ion pumps; transporting molecules, amino acids, and proteins; and synthesizing proteins and lipids. Most ATP formation comes from the metabolism of glucose in the presence of oxygen; when oxygen is limited, ATP production from glucose is much less and lactic acidosis occurs.
 When sufficient energy is not present, neurons die by either apoptotic or necrotic mechanisms. Apoptosis is programmed cell death that leads to normal cell death during development and excessive cell death with energy deprivation due to ischemia. Neurotrophic factors inhibit apoptotic cell death and enhance survival. Necrosis occurs when energy deprivation is more severe due to more prolonged or extreme ischemia; this leads to inflammation and more extensive damage of the surrounding brain tissue.
When sufficient energy is not present, neurons die by either apoptotic or necrotic mechanisms. Apoptosis is programmed cell death that leads to normal cell death during development and excessive cell death with energy deprivation due to ischemia. Neurotrophic factors inhibit apoptotic cell death and enhance survival. Necrosis occurs when energy deprivation is more severe due to more prolonged or extreme ischemia; this leads to inflammation and more extensive damage of the surrounding brain tissue.
 CBF is highly regulated and coupled to metabolism. Blood flow is increased when carbon dioxide (CO2) is increased (may be due to increased metabolism or hypoventilation) and reduced when CO2 is decreased (may be due to decreased metabolism or hyperventilation); these changes in CBF are due to changes in brain pH. If blood pressure changes within a range (for normal patients 50 to 150 mm Hg perfusion pressure), the blood flow in the brain remains constant; this is termed autoregulation.
CBF is highly regulated and coupled to metabolism. Blood flow is increased when carbon dioxide (CO2) is increased (may be due to increased metabolism or hypoventilation) and reduced when CO2 is decreased (may be due to decreased metabolism or hyperventilation); these changes in CBF are due to changes in brain pH. If blood pressure changes within a range (for normal patients 50 to 150 mm Hg perfusion pressure), the blood flow in the brain remains constant; this is termed autoregulation.
 Cerebrospinal fluid (CSF) is mainly formed in the choroid plexus of the cerebral ventricles and is absorbed in the arachnoid villi. Increased formation or decreased absorption of CSF can increase the volume of fluid in the cranium and lead to high ICP. Brain tissue swelling (cytotoxic edema) and/or increased extracellular fluid due to vasogenic edema or a bleed into the brain can also increase the volume in the cranium and lead to high ICP. High ICP can lead to decreased vascular perfusion of the brain or brain herniation, either of which can cause severe brain damage.
Cerebrospinal fluid (CSF) is mainly formed in the choroid plexus of the cerebral ventricles and is absorbed in the arachnoid villi. Increased formation or decreased absorption of CSF can increase the volume of fluid in the cranium and lead to high ICP. Brain tissue swelling (cytotoxic edema) and/or increased extracellular fluid due to vasogenic edema or a bleed into the brain can also increase the volume in the cranium and lead to high ICP. High ICP can lead to decreased vascular perfusion of the brain or brain herniation, either of which can cause severe brain damage.
I. BRAIN AND SPINAL CORD PHYSIOLOGY
To understand the effects of anesthesia and surgery on the nervous system, one needs to know basic cellular neurophysiology as well as organ-level physiologic function. This section briefly describes the basic principles of neurophysiology.
A. Cellular neurophysiology. The basic properties of neuronal excitability are due to a change in the membrane potential so that a threshold is reached and the neuron fires an action potential. This propagates to the axon terminal and leads to neurotransmitter release, which influences the membrane potential of a second neuron.
1. Membrane potentials are voltages measured across the cell membrane due to an unequal distribution of ions across that membrane. A combination of the equilibrium potential for a particular ion and the membrane’s conductance (permeability) for that ion determines its contribution to the membrane potential.
a. The equilibrium potential (E) for an ion can be calculated using the Nernst equation if the intra- (for potassium, Ki) and extracellular (K0) concentrations of that ion are known. For an ion with a single positive charge, the equation simplifies to EK =−61 log[Ki/K0] at 37°C. Under normal physiologic conditions in the nervous system, the equilibrium potential for potassium (K) is approximately −90 mV and for sodium (Na) +45 mV.
1. The relative conductance of the neuronal membrane to different ions determines the membrane potential. This conductance (g) for the different ions varies with conditions, input to the specific neuron, and time. The membrane potential of a neuron at any point in time can be described by the following equation:
| (1) |
where gx is the conductance for ion x and Ex is the equilibrium potential for that ion. The resting membrane potential for a neuron is approximately −70 mV, which is closer to the EK (−90 mV) than the ENa (+45 mV) because gK is much greater than gNa in resting (unexcited) neurons.
2. There are concentration-dependent and electrical field–dependent forces acting on ions; the sum of these forces determines whether the net movement of a particular ion will be into or out of that neuron. This is referred to as the electrochemical gradient for that ion.
2. Action potentials are regenerative changes in a neuron’s membrane potential due to excitation of the neuron so that its membrane potential depolarizes past a certain threshold. During an action potential, a rapid initial increase in the gNa is followed by a return to baseline and a slower increase in the gK. These conductance changes lead to a short and rapid depolarization followed by a repolarization. This is sometimes followed by a hyperpolarization after the action potential (Fig. 1.1).
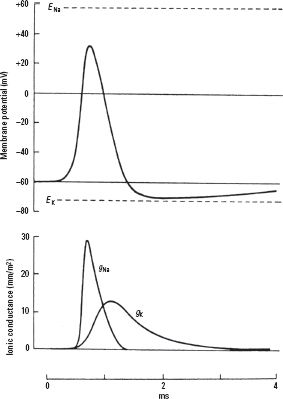
FIGURE 1.1 Changes in the membrane potential and the sodium and potassium conductances (gNa and gK) during an action potential. ENA and EK are the sodium and potassium equilibrium potentials. (From Aidley DJ. The Physiology of Excitable Cells. Cambridge: Cambridge University Press, 1989:65, as redrawn from Hodgkin AI, Huxley AT. A quantitative description of membrane and excitation in nerve current and its application to conduction. J Physiol. 1952;117:500–544.)
a. The Na conductance changes are due to the opening of a protein channel in the membrane that is selectively permeable to Na ions. This channel has one activation and one inactivation gate, both of which must be in the open configuration if the channel is to allow Na through it. The rapid opening and closing of this channel are in part responsible for the brief duration of the action potential.
b. At rest, more K than Na channels are open. With the action potential, more Na channels open so that the gNa is greater than the gK and the neurons depolarize. The depolarization causes a slow opening of K channels, increasing gK and leading to a repolarization (gK > gNa). In the period after the action potential, when the Na channels have become inactivated, the increased gK can actually cause a hyperpolarization below the resting potential; this so-called afterhyperpolarization is frequently found in neurons.
3. Synaptic transmission is the process by which one neuron (presynaptic neuron) influences the membrane potential and thereby the action potential generation in a second neuron (postsynaptic neuron). The axon terminals of a neuron contain vesicles with neurotransmitter molecules in them. When a terminal is depolarized, voltage-sensitive Ca channels open, increasing the Ca concentration in the terminal. This Ca increase causes the vesicles to release neurotransmitter into the synaptic cleft. The transmitter diffuses across the synapse and binds to specific receptors on the postsynaptic neuron. Their effect on the postsynaptic neuron depends on the ion channels that are opened or the biochemical processes that are altered by the activation of that receptor.
a. Ionotropic receptor activation opens membrane channels for certain ions that can either hyperpolarize or depolarize the postsynaptic neuron, making it less or more likely to fire an action potential.
b. Metabotropic receptors can activate second messengers that alter neuronal biochemical parameters. This can lead to long-term changes in a neuron’s activity.
4. Glutamate is a major excitatory neurotransmitter in the central nervous system (CNS). Its activation depolarizes neurons, increasing the number of action potentials generated.
a. There are three major ionotropic glutamate receptors: alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate, and N-methyl D-aspartic acid (NMDA). The AMPA and kainate receptors are attached to ion channels that allow Na and K to pass through them; a small number of AMPA receptors are also permeable to Ca. The NMDA channels, activated when neurons are already depolarized, are permeable to Na, K, and Ca. Activation of NMDA channels has been associated with long-term changes in neuronal activity that may be cellular correlates of learning and memory. Overactivation of glutamate receptors has been associated with neuronal injury from epilepsy, trauma, and ischemia.
b. Metabotropic receptors are also activated by glutamate. These receptors act via guanosine-5′-triphosphate (GTP)-binding proteins (G proteins) to affect ion channels or second-messenger pathways (e.g., cyclic 3′,5′adenosine monophosphate [cAMP], inositol 1,4,5-trisphosphate), which in turn can alter ionic conductance, cell Ca levels, and other biochemical changes. The effect of metabotropic receptor activation is longer in duration than that of ionotropic receptor activation.
5. Gamma-aminobutyric acid (GABA) and glycine are major inhibitory neurotransmitters in the CNS. Their activation hyperpolarizes neurons, decreasing the number of action potentials generated. Inhibition is important for the brain and spinal cord to function. When inhibition is substantially reduced, seizures can occur and lead to complete loss of function and permanent brain damage.
a. GABA is a major inhibitory transmitter in the brain and spinal cord. The GABAA receptor contains a chloride (Cl) channel that is opened when GABA binds. This activity is augmented by benzodiazepines, volatile anesthetics, and barbiturates. The GABAB receptor acts via a second messenger to open K channels.
b. Glycine is a major inhibitory transmitter in the spinal cord that opens a ligand-gated Cl channel. Strychnine is an antagonist of the glycine receptor and causes convulsions and even death.
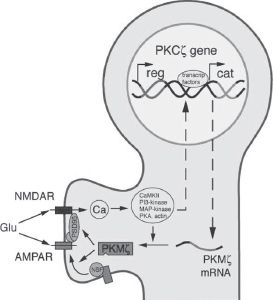
6. Long-term potentiation (LTP) is a cellular property associated with learning and memory and requires protein kinase activity. This is illustrated in Figure 1.2.
a. LTP can be caused by a high-frequency train of stimuli and increases the response of postsynaptic neurons to subsequent single stimuli.
1. The high-frequency stimuli cause an increase in NMDA receptor activity and cytosolic Ca levels. This activates kinases and increases AMPA channels in the membrane.
b. LTP is considered a cellular neurophysiologic correlate of learning and memory.
c. Protein kinase M zeta (PKM zeta) is important for maintenance of LTP and learning and memory.
1. Its concentration increases following LTP.
2. A peptide inhibitor of PKM zeta prevented the maintenance of LTP.
3. This inhibitor when infused in the brain caused animals to forget a previously-learned task.
7. Anesthetics, like neurotransmitters, can act directly on ion channels (ionotropic actions) and/or alter cell metabolism and signaling pathways (metabotropic actions).
a. Ionotropic mechanisms of anesthetics
1. Anesthetics bind to GABAA receptors and enhance the inhibition caused by the activation of their associated Cl channels.
a. Barbiturates, volatile anesthetic drugs, propofol, and benzodiazepines enhance GABAA transmission.
2. Anesthetics can bind to glutamate channels and reduce their activity.
a. Barbiturates, volatile anesthetic drugs, and propofol reduce glutaminergic transmission and thereby excitability.
b. Metabotropic effects of anesthetics on cellular signaling pathways
1. Anesthetics can enhance the activity of protein kinases that phosphorylate and thereby alter the activity of other proteins in the cell.
Protein kinase C (PKC) and PKM zeta can affect cell metabolism and ion channel activity. These kinases also appear to play a role in anesthetic preconditioning protection of neurons.
2. Anesthetics can increase Ca concentrations in the cytosol, which can alter cell signaling mechanisms and biochemical pathways.
3. Volatile anesthetic drugs such as isoflurane and sevoflurane have been demonstrated to have metabotropic effects.
c. Anesthetics can interfere with learning and memory.
8. Active transport maintains the ionic concentrations required for neuronal function. There is a constant leak of ions down their electrochemical gradients. If not corrected, this leak leads to a loss of these ion gradients. Ion pumps use energy to maintain the ion concentrations necessary for neuronal viability. During ischemia, a decrease in energy production and a loss of ion gradients occur (Fig. 1.2).