Structures of neurokinin-1 (NK1)-receptor antagonists according to the PubChem database. (a) CP96345 (PubChem CID 104943). The binding elements for NK1 receptor are shown in dotted circles: (1) quinuclidine group; (2) benzhydryl group; and (3) o-methoxybenzylamine group. (b) Aprepitant (PubChem CID 151165) with the morpholine ring highlighted. (c) Fosaprepitant (PubChem CID 219090) with the phosphate group on the oxotriazolyl ring shown. (d) Casopitant (PubChem CID 9917021) with the phenylpiperazine ring shown. (e) Netupitant (PubChem CID 6451149) showing the aryl-isoxazol group. (f) Rolapitant (PubChem CID 10311306) with the phenylglycinol group highlighted.
Using CP96345 and CP99994 as templates for drug design, Merck synthesized the first commercially available NK1 antagonist – aprepitant[23,24]. In this drug, the piperidine ring was replaced by a morpholine ring to improve oral absorption, and an electron-withdrawing group was attached to the piperidine nitrogen to avoid calcium-channel activation. Finally, metabolic stability was achieved by methylation of alpha-carbon and fluorination of the aromatic ring. Recently, a water-soluble prodrug of aprepitant was produced (fosaprepitant) by phosphorylation of the oxotriazolyl ring and has been given intravenously[25].
In parallel, several pharmaceutical companies were interested in developing NK1-receptor antagonists for a variety of indications. GlaxoSmithKline found that molecules with phenylpiperazine rings bound to the NK1 receptor readily[26]. Among these compounds, casopitant was the most active. The drug was tested in a series of phase III clinical trials for preventing nausea and vomiting after chemotherapy and surgery. However, further development was discontinued in 2009 because substantial work was needed to fulfill regulatory requirements. Netupitant was developed by Roche, which was modified from aryl-isoxazoles and recently approved for the prevention of chemotherapy-induced nausea and vomiting (CINV)[27]. Rolapitant was developed by Schering-Plough, which is a phenylglycinol derivative and is currently under evaluation by a number of regulatory authorities[28].
Effects of NK1-receptor antagonists
NK1 receptor blockade demonstrates profound anti-inflammatory effects in cellular experiments[9]. Depending on the sites of action, NK1-receptor antagonists produce diverse effects in different organ systems. Table 8.2 shows a summary of the therapeutic effects of NK1-receptor antagonists observed in preclinical studies.
| Effects | Preclinical study | Clinical trials |
|---|---|---|
| Analgesia | ++ | − |
| Antiemetic | +++ | +++ |
| Reduce bronchial hypersensitivity | ++ | − |
| Reduce gut hypermotility | ++ | ± |
| Antipruritic | + | ? |
| Reduce detrusor hyperreflexia | ++ | ± |
| Reduce postoperative fibrous adhesion | ++ | ± |
| Antidepressant | ++ | ± |
| Antisepsis | ++ | ? |
| Antineoplastic | ++ | ? |
+++, marked effect; ++, moderate effect; −, no effect; ±, equivocal data; ?, no clinical trial has been performed.
These therapeutic effects can be summarized as follows:
(1) Centrally and peripherally acting NK1 antagonists were effective in attenuating nociceptive responses in different experimental pain models, particularly those related to nerve injury and tissue inflammation, among different species (e.g., gerbils, rats and guinea pigs)[29,30].
(2) In dogs and ferrets, NK1-receptor antagonists were highly effective against all forms of emetic stimuli[31,32].
(3) Dual selective NK1/NK2 receptor antagonist (DNK333) reduced airway responsiveness to allergen challenge in guinea pig[33].
(5) NK1-receptor antagonist reduced scratching behavior in a mouse model of dermatitis[36].
(6) NK1-receptor antagonist (TAK637) reduced detrusor hyperreflexia in a guinea pig model of capsaicin-induced bladder contraction[37].
(7) NK1 antagonists produced anxiolytic-like effect in cats[38] and attenuated vocalization due to maternal separation in guinea pigs; these data suggested a potential antidepressant effect[39].
(8) Intraperitoneal aprepitant reduced fibrous adhesions following experimental laparotomy in rats[40].
(9) NK1 antagonists (SR140333) reduced leukocyte recruitment and prevented lung injury in a Swiss mouse model of polymicrobial sepsis[41].
(10) In cellular experiments, NK1-receptor antagonist (L733060) suppressed proliferation of colonic carcinoma[42].
Despite the benefits shown in cell and animal experiments, replication of these findings in adequately designed clinical trials has been largely disappointing. Currently, apart from the management of nausea and vomiting, there is a lack of clinical evidence to show that NK1 antagonists could be used to alleviate pruritus, bronchial hypersensitivity[43] or symptoms associated with irritable bowel syndrome and overactive bladder[34,44]. There is also a lack of efficacy for NK1 antagonists to reduce pain[45]. In a small trial of 78 patients having third molar extraction, an infusion of CP99994 (750 μg/kg) prior to surgery produced limited analgesia that lasted <90 min. More importantly, pain relief was inferior to oral ibuprofen 600 mg[46]. In another study evaluating the effects of aprepitant on PONV in 60 women having gynecologic laparoscopic surgery, consumption of postoperative analgesics was reduced by half in patients receiving aprepitant, but there was no difference in pain score between groups[47]. Other studies have also failed to demonstrate useful analgesia with NK1 antagonists in migraine[48,49] and pain associated with osteoarthritis and diabetic neuropathy[43,50,51]. Similarly, NK1 antagonists did not appear to relieve symptoms of major depression or other affective and addictive disorders[52]. There is currently no study evaluating the effects of NK1-receptor antagonists in sepsis or cancer growth.
It is unclear why human studies have failed to confirm the findings in preclinical experiments. Investigators have speculated that differences in receptor function and distribution among species may have contributed to the discrepancy[9,45,53]. Nevertheless, the lack of effect of NK1-receptor antagonists for other indications in humans added to the safety profile of these compounds for PONV.
NK1-receptor antagonists for managing nausea and vomiting
Currently, clinical application of NK1-receptor antagonists is largely limited to the management of nausea and vomiting. In particular, research has focused on the use of these compounds to control CINV and those following anesthesia and surgery.
Chemotherapy-induced nausea and vomiting
In an early trial, a single dose of fosaprepitant (60 or 100 mg) given intravenously was found to be ineffective to prevent acute (<24 h) vomiting following cisplatin treatment, but the control for delayed (day 2–7) nausea and vomiting was superior in the fosaprepitant group compared with ondansetron[54]. Subsequent trials, however, showed that the addition of NK1-receptor antagonists (vofopitant[55], CP122721[56], ezlopitant[57], aprepitant[58–64] and casopitant[65–68]) to ondansetron or granisetron (5-HT3 antagonists) and dexamethasone (i.e., triple therapy) increased the complete response rate (defined as no vomiting and no need for rescue antiemetic) from 42–61% to 51–86% (relative increase of 18–47%) in both acute and delayed phases. These data demonstrated that multimodal therapy using a combination of antiemetics is required when dealing with intense emetogenic stimuli. Based on this idea, a longer-acting NK1-receptor antagonist (netupitant) is currently marketed as a combination pill, which included another long-acting 5-HT3-receptor antagonist (palonosetron) in a fixed-dose ratio. In a series of phase III studies, NEPA (netupitant 300 mg and palonosetron 0.5 mg) was highly effective and prevented vomiting in 98.5% of patients receiving cisplatin chemotherapy in the acute phase and 91.9% in the subsequent 4 days[69]. In another trial, NEPA with dexamethasone was compared with palonosetron and dexamethasone in patients having moderately emetogenic chemotherapy. The complete response rate was increased from 66.6% to 74.3%[70]. Finally, in patients having repeated cycles of chemotherapy, prophylaxis with NEPA and dexamethasone performed similarly to that of aprepitant regimen with an overall complete response rate >76%[71]. The current guidelines from the European Society of Medical Oncology and the Multinational Association of Supportive Care in Cancer recommend the addition of an NK1-receptor antagonist to a 5-HT3-receptor antagonist and dexamethasone for the prophylaxis of nausea and vomiting after highly emetogenic chemotherapy[72]. At present, aprepitant, fosaprepitant and NEPA are approved for managing CINV.
Postoperative nausea and vomiting
Building on the success of NK1-receptor antagonists in CINV, investigators moved quickly to evaluate the impact of these drugs in preventing PONV. The first clinical study was a small randomized trial in 36 women having major gynecologic surgery. Intravenous vofopitant (GR205171) 25 mg compared with placebo reduced the incidence of vomiting, severity of nausea and the requirement for rescue antiemetic[73]. In another study using CP122721 100–200 mg orally in women having abdominal hysterectomy (n = 243), the effect was striking. During the entire 72-h study period, the incidence of vomiting and retching was reduced from 80% in the placebo group to 46% in patients receiving CP122721, with a number needed-to-treat of 3 (95% confidence intervals (CI), 1.9–6.9)[74]. The drug also compared favorably with ondansetron. Over the first day after surgery, the incidence of vomiting in CP122721 group was 11.1% and was significantly lower than that in the ondansetron group (46.2%, P = 0.002). Unfortunately, both drugs were not further developed for commercialization.
Two larger-scale, multicenter parallel studies evaluated the efficacy of aprepitant for the prevention of PONV in patients having major abdominal surgery[75,76]. Based on an identical protocol, the two trials compared aprepitant 40 and 125 mg with ondansetron 4 mg as active control. Both trials reported a profound reduction in postoperative vomiting (POV) after aprepitant treatment compared with ondansetron (11.6% versus 27.6%; odds ratio (OR), 0.34; 95% CI, 0.26–0.45; P < 0.001)[77]. However, the effect of aprepitant on postoperative nausea was less obvious. Sixty-two percent of patients in the aprepitant group reported nausea in the first day after surgery and 79% of these patients had significant nausea with a visual nausea score >4 out of 10. Aprepitant (40 mg) seemed to produce a similar effect compared with aprepitant 125 mg (Figure 8.2)[76], but it is unclear whether bigger doses of aprepitant would produce better protection. Further dose–response studies may be required to define the optimal dose of aprepitant for preventing PONV. When compared with ondansetron, there was only a marginal effect for aprepitant to prevent nausea (OR, 0.81; 95% CI, 0.65–1.00; P = 0.05)[75–77]. The preferential effect of aprepitant on POV was again demonstrated in subsequent trials of patients having craniotomy[78,79], ambulatory procedures[80], gynecologic[81,82], rhinolaryngologic[83] and bariatric surgery[84]. Overall, the number of patients needed to be treated with aprepitant, instead of 5-HT3-receptor antagonists, in order to avoid an episode of POV was 6 (95% CI, 4.9–7.6). In contrast, the corresponding number for avoiding nausea was 12 (95% CI, 7.2–23.8).
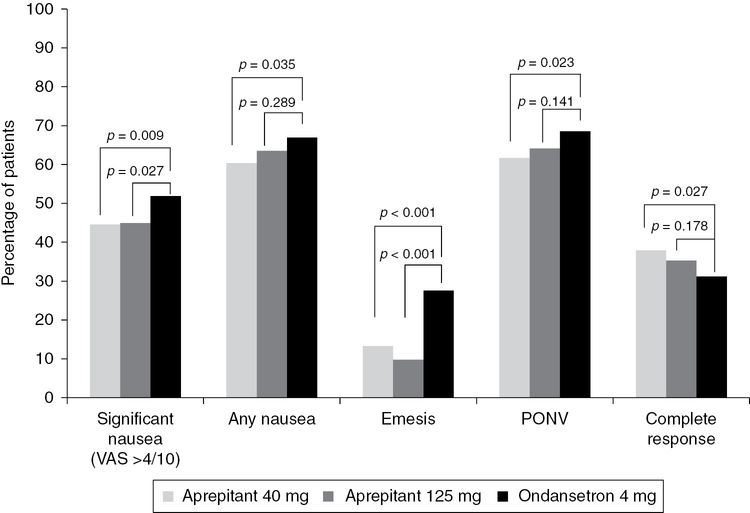
Percentage of patients reporting significant nausea, vomiting, postoperative nausea and vomiting (PONV) and complete response in trials comparing aprepitant 40 mg (n = 541) and 125 mg (n = 532), and ondansetron 4 mg (n = 526). VAS, visual analog scale.
Similar results were reported for casopitant. In 1,700 women at high risk of PONV, the incidence of POV was reduced from 28% in the ondansetron group to 8.8% in those receiving casopitant 50–150 mg (OR 0.25; 95% CI, 0.17–0.35; P < 0.001)[85,86]. The impact of casopitant on nausea remained suboptimal. Finally, in 619 patients having open abdominal surgery, rolapitant (a longer-acting NK1-receptor antagonist) at doses of 70 or 200 mg prevented PONV between 24–120 h after surgery compared with ondansetron (25.6% versus 38.5%, P = 0.03)[87]. In both groups, >83% reported nausea during the study period.
Current data suggest that NK1-receptor antagonists are potent antiemetics that could reduce the incidence of POV to ≤10%, even in patients at high risk of PONV. However, their effect on postoperative nausea is limited. Interestingly, vofopitant is also ineffective in motion sickness, a condition where nausea is a prominent feature[88]. Currently, only aprepitant is approved for the prevention of PONV.
Pharmacokinetics of NK1-receptor antagonists
The pharmacokinetic parameters of NK1-receptor antagonists are shown in Table 8.3.
| Aprepitant | Fosaprepitant | Casopitant | Netupitant | Rolapitant | |
|---|---|---|---|---|---|
| Bioavailability | 60–65% | ̶ | 83% | High | High |
| Time to peak absorption (h) | 3–4 | ̶ | 0.5–1.5 | 5 | 2–3 |
| Protein binding | >95% | ̶ | >99% | 99% | NA |
| Volume of distribution (L/kg) | 1 | 0.1 | 3–4 | 30–40 | NA |
| Elimination half-life | 9–13 h | 2.3 min | 8–15 h | 96 h | 180 h |
| Clearance (mL/min) | 60–90 | ~ 60 | 200 | 300 | NA |
| Metabolism pathway | Liver, oxidation by CYP3A4 to weakly active metabolites | Blood and tissue by ubiquitous phosphatase, convert to aprepitant | Liver, oxidation and N-dealkylation by CYP3A4 to inactive metabolites | Liver, oxidation by CYP3A4, metabolites capable to bind to NK1 receptor | Liver, oxidation by CYP3A4 to inactive metabolites |
| Dose reported for prevention of nausea and vomiting | |||||
| PONV | 40 mg orally, 3 h prior to surgery | No data | 50 mg orally, 1 h prior to surgery or 30 mg IVI at induction of anesthesia | No data | 50–200 mg, 0.5 h prior to surgery |
| CINV | 125 mg before and 80 mg daily for day 1 and 2 | 115 mg produce similar effect as oral aprepitant 125 mg | 150 mg orally before and 50 mg daily for day 1 and 2 | 300 mg orally before chemotherapy | 200 mg orally before chemotherapy |
CYP, cytochrome; CINV, chemotherapy-induced nausea and vomiting; IVI, intravenous infusion; NA, not available; PONV, postoperative nausea and vomiting.
Aprepitant
Aprepitant is a highly lipophilic base with a pKa value of 9.7 (at pH 2–12). Given the lipophilicity, aprepitant cannot be dissolved in aqueous solution for intravenous (IV) injection. For the same reason, gut absorption of the drug is highly dependent on dietary fat. In order to increase bioavailability, current formulation reduces particle size to nanoscale (<150 nm)[89]. The nanoparticles increase surface exposure by 3–4-fold and oral bioavailability to about 60–65%. Drug absorption of the nanoparticle is independent of food intake. In previous studies, peak plasma concentrations were achieved at 3 and 4 h following oral administration of aprepitant 40 and 125 mg, respectively, and the corresponding plasma concentrations were 700 ng/mL and 1400 ng/mL[90,91]. At these plasma concentrations, NK1 receptors in the brain were estimated to be >99% occupied.
Aprepitant is largely bound to plasma proteins (>95%) with a steady-state volume of distribution of about 1 L/kg[91]. Aprepitant is largely metabolized by microsomes (CYP3A4 isoenzyme) in the liver, where it is oxidized at the morpholine ring to produce a number of inactive metabolites. Plasma clearance is in the range of 60–90 mL/min, resulting in a terminal half-life of 9–13 h[91]. Renal impairment has no apparent effect on drug elimination. No study has evaluated the effect of liver failure on the metabolism of aprepitant.
Aprepitant is a substrate as well as a mild-to-moderate inhibitor of CYP3A4. There have been concerns that aprepitant may alter the pharmacokinetics of concomitantly administered drugs that are metabolized by CYP3A4, and hence resulting in adverse drug interactions. CYP3A4 inhibition appears to affect CYP3A4 in the gut more than that in the liver. In this respect, bioavailability of orally administered midazolam was substantially increased (3.3-fold) by coadministration of aprepitant for 5 days[92]. In contrast, plasma midazolam concentration was modestly increased by 25% when the drug was given intravenously[93]. Currently, the only notable interaction for aprepitant is coadministration of dexamethasone or methylprednisolone. When aprepitant was given for 3 days according to standard regimen for CINV, plasma concentrations of the two corticosteroids were increased by greater than twofold[94]. Currently, no study has evaluated the clinical relevance of this drug interaction. Nevertheless, it is recommended that the dosage of coadministered dexamethasone or methylprednisolone should be halved[95]. Aprepitant also induces CYP2D6 and may decrease the effects of warfarin and oral contraceptive drugs. Therefore, monitoring of anticoagulation effect has been recommended[96]. Aprepitant has no apparent effect on 5-HT3 receptor antagonists[95]. It should be noted that all these drug interaction studies were performed in subjects receiving multiple and large doses of aprepitant. A single dose of aprepitant 40 mg given before surgery for preventing PONV is unlikely to produce significant drug interaction.
Fosaprepitant
Fosaprepitant is a water-soluble N-phosphoryl derivative of aprepitant[25]. As a prodrug, fosaprepitant is rapidly converted to its parent compound by ubiquitous phosphatases after IV administration. In volunteers receiving fosaprepitant 115 mg, the elimination half-life of fosaprepitant was 2.3 min, so that all drug was cleared from plasma within 30 min of injection[97]. There is hardly any tissue distribution and the volume of distribution is estimated to be about 5 L[97,98]. Fosaprepitant is capable of binding to NK1 receptors but with much lower affinity (binding affinity (IC50) values for aprepitant and fosaprepitant of 0.09 and 1.2 nM, respectively)[25]. Therefore, the effect of fosaprepitant can be attributed entirely to aprepitant.
Casopitant
Casopitant is a substituted piperazine derivative. It has been formulated as a powder and in film-coated tablets for oral administration, and has been dissolved in sodium citrate buffer for IV injection. After oral ingestion, casopitant is rapidly absorbed with a bioavailability in excess of 83% and is not affected by dietary factors. Peak plasma concentration is reached in 30–90 min[99]. Casopitant crosses the blood–brain barrier freely. In volunteers receiving multiple doses of oral casopitant, a steady-state plasma concentration >20 ng/mL was associated with an NK1 receptor occupancy of >95%[100]. It should be noted that a single dose of casopitant of 50 mg produced a peak plasma concentration >100 ng/mL. Casopitant is highly bound to plasma proteins (>99%) with a volume of distribution of 2–3 L/kg. It is primarily metabolized (oxidation and N-dealkylation) in the liver by CYP3A4 to produce a large number of inactive metabolites. The total clearance is about 200 mL/min[101]. The terminal half-life varies between 8.8 and 15.1 h. Casopitant is a mild-to-moderate inhibitor of CYP3A4, and therefore shares the same concerns of potential drug interactions with aprepitant. Current data suggest that a single dose of casopitant has little effect on the pharmacokinetics of coadministered 5-HT3 receptor antagonists, dexamethasone and midazolam[99].
Netupitant
Netupitant is a long-acting NK1-receptor antagonist (elimination half-life of 96 h). It is a lipophilic molecule, currently formulated as a combination pill with another long-acting 5-HT3 receptor antagonist – palonosetron (half-life of 44 h). Following oral administration, absorption is almost complete, producing peak plasma concentration at about 5 h. Netupitant is highly bound to plasma proteins (99%), with a volume of distribution of 30–40 L/kg. It is metabolized in the liver by CYP3A4. In contrast to the other NK1-receptor antagonists, the resulting metabolites were capable of binding to NK1 receptors. Current data show that netupitant has no effect on the pharmacokinetics of palonosetron, but the dosage of dexamethasone should be reduced[102].
Rolapitant
This is a very long-acting NK1-receptor antagonist, with an elimination half-life of 180 h. It is rapidly absorbed with the peak plasma concentration occurring at 2–3 h after oral administration. Rolapitant is metabolized in the liver but does not inhibit liver enzymes, including CYP3A4. Therefore, risk of drug interaction is considered low.
Taken together, NK1-receptor antagonists appear to be very safe. Reported adverse events in clinical trials were similar to those in the control groups (usually ondansetron)[103]. The most common side effects were headache and constipation.
Novel antiemetic: the antipsychotic amisulpride
Several antipsychotics have been tried in the management of nausea and vomiting. Amisulpride, a second-generation antipsychotic, has emerged as a novel antiemetic for managing PONV. As a substituted benzamide, amisulpride preferentially blocks dopamine (D2 and D3) receptors. At therapeutic dosage for treatment of psychosis (400–1,200 mg/day), there is no interaction with adrenergic, 5-HT and cholinergic receptors[104]. Consequently, the risk of extrapyramidal movement, sedation and cardiac arrhythmia with prolonged QTc intervals were lower compared with first-generation agents such as haloperidol[105].
The efficacy of amisulpride to prevent PONV has been studied in a recently published randomized controlled trial[106]. In this trial, 215 patients at risk of PONV were randomized to receive placebo or IV amisulpride 1, 5 or 20 mg. Interestingly, only amisulpride 1 or 5 mg reduced the risk of PONV during the first 24 h after surgery. The numbers needed-to-treat were 5 (95% CI, 2.7–21.6) for PONV and 5 (95% CI, 2.4–15.8) for nausea. These are encouraging data and further trials are required to define the role of low-dose amisulpride as an antiemetic after surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





