Fig. 31.1
The Bion implantable neurostimulator device. For investigational use only. Not approved for sale in the United States
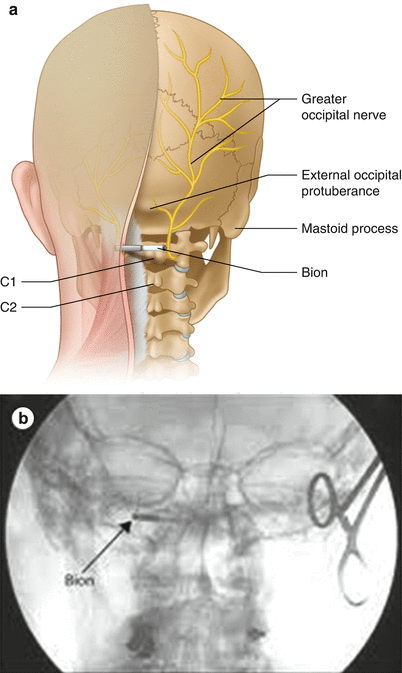
Fig. 31.2
(a) The implant location of the Bion in the occipital region for the treatment of chronic headaches. (b) AP radiograph of an implanted Bion device
In another study, nine patients had the Bion implanted in the occipital region for chronic migraine, hemicranias continua, or cluster headaches [2]. Of these patients, eight completed the 12-month follow-up with seven of eight obtaining fair or better results (at least a 25 % reduction) in reducing their disability. Six patients suffering from hemicrania continua (HC) had the Bion implanted in a crossover study [3]. In long-term follow-up, four of six patients reported substantial (≥80 %) reduction in pain severity and another reported a 30 % reduction. One patient reported a 20 % increase in pain. Although these studies examine small numbers of patients without randomization, they appear to demonstrate some promise of small implantable neurostimulators for treating pain, particularly in chronic headache disorders. In addition to evidence of its efficacy, these studies found the Bion to generally be safe.
31.3 Bioness StimRouter
The Bioness StimRouter is a single percutaneously inserted lead that has three tightly spaced contacts on its head and a radiofrequency receiver on its tail [4]. This device is currently being studied in a clinical trial for post-traumatic or postsurgical neuropathic pain of a single peripheral nerve. A patient wears an external patch that acts as the generator and transmits radiofrequency energy transcutaneously to power the device. The system offers several advantages over existing PNS devices. The device implantation is simple and minimally invasive. The device’s small size avoids the discomfort to the patient of a larger implanted device. The external pulse transmitter avoids the need for an implanted battery and subsequent surgical procedures for battery replacement. Disadvantages may include the relatively small surface area of the active contacts on its head, the fact that a patient must wear an external patch whenever she or he want the device to be operational, and the possibility of skin erosion from the adhesive of the patch.
The StimRouter was tested in an open-label study with eight patients suffering from carpal tunnel syndrome and chronic pain despite carpal tunnel release and medication [5]. The StimRouter was implanted along the median nerve of eight patients (six patients receiving a single StimRouter and two receiving bilateral device implantations). Patients received 5 days of stimulation with the device, experiencing a mean pain reduction of 37 % from baseline on day 5 and reduced their oral opioid medication use while receiving stimulation. No adverse events were reported.
31.3.1 Lead
The StimRouter consists of a single 15-cm lead with a body diameter of 1.2 mm. The lead is made from a platinum-iridium alloy covered in silicone tubing. One end of the lead contains a single receiver; the other end contains three stimulating electrodes. The stimulating end also has a four-pronged polypropylene anchor. This anchor helps ensure that the lead stays in place once the device has been implanted. The lead is flexible, making lead migration and fracture less likely.
31.3.2 External Pulse Transmitter
The StimRouter lacks an IPG. Instead the device is powered by an external pulse transmitter (EPT). This EPT connects to a disposable patch with an adhesive electrode hydrogel. This user patch must be replaced every 2–3 days. The EPT and patch are placed on the skin over the implanted lead. The EPT and user patch transmit transdermal electrical stimulation that is picked up by the lead’s receiver electrode and transmitted through the stimulation electrodes to the target nerve. A rechargeable battery built into the EPT’s case provides power.
31.3.3 Patient Programmer
The StimRouter can be turned on or off and the device settings adjusted with a patient programmer. This programmer wirelessly communicates with the EPT. During office visits a clinician programmer is used to set device parameters for delivering effective stimulation. The patient programmer allows the patient to make finer adjustments to the intensity of the stimulation and to switch among up to eight stimulation programs.
31.3.4 Target Patients for the StimRouter
The StimRouter is intended to treat patients with peripheral mononeuropathies. These include post-traumatic neuropathies, postsurgical neuropathies, and other neuropathies. Patients who experience moderate to severe chronic pain limited to a single peripheral nerve may be good candidates for this treatment. Patients with appropriate neuropathies may be identified by electromyography (EMG) testing or diagnostic nerve blocks using local anesthetic. Patients who receive significant temporary relief from a nerve block of a single peripheral nerve may get relief from a PNS such as the StimRouter (Fig. 31.3).
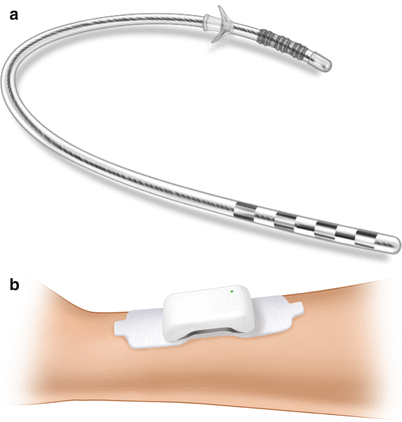

Fig. 31.3
(a) The Bioness StimRouter peripheral neurostimulator lead. For investigational use only. Not approved for sale in the United States. (b) The Bioness StimRouter peripheral neurostimulator EPT and electrode patch. For investigational use only. Not approved for sale in the United States
Possible target nerves for PNSs include:
Arms: axillary, radial, ulnar, median, digital
Legs: femoral, sciatic, saphenous, tibial, sural, deep peroneal, common peroneal, superficial peroneal, lateral cutaneous, posterior cutaneous
Trunk: suprascapular, thoracic, intercostal, ilioinguinal, iliohypogastric, gluteal
31.3.5 Lead Implantation
The StimRouter lead is implanted in a 30- to 45-min outpatient procedure. Conscious sedation is generally used during this procedure.
Get Clinical Tree app for offline access

1.
Inserting the stimulation probe (Fig. 31.4). An initial 1-cm incision is made approximately 8–10 cm from the target nerve under local anesthetic. A stimulation probe is inserted into the incision and guided to the nerve. This can be performed under ultrasound guidance. Electrical stimulation is sent through the stimulation probe to test for paresthesia in the area of the patient’s typical pain.

Fig. 31.4
Inserting the stimulation probe for Bioness StimRouter placement
2.
Inserting the loader (Fig. 31.5). Once paresthesia is achieved the target area, a loader is inserted over the stimulation probe.
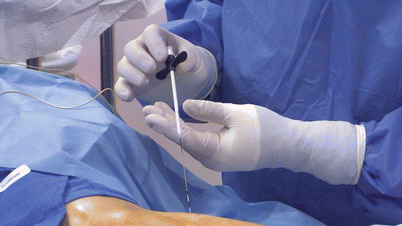

Fig. 31.5
Inserting the loader for Bioness StimRouter placement
3.
Inserting the lead into the loader (Fig. 31.6). The probe is then removed and the lead is placed inside the loader. The rubber ring on the reed containing the lead is rolled back to expose the electrodes on the lead. The lead is then used to deliver stimulation.