Fig. 27.1
Anatomy of the knee and its innervation
27.3 Patient Selection
The ideal patient for consideration of PNS to treat knee pain can be identified by several characteristics. The patient should complain of burning knee pain in the distribution of a definable nerve or nerve branch. The examination should demonstrate evidence of abnormal nerve function, either nerve hypersensitivity or a loss of sensation. Attempts to treat the pain with physical medicine, reasonable oral and topical medications, and other conservative measures should be unsuccessful or unacceptable. The predictive ability of a nerve block to determine a proper candidate has not been proven in any prospective study, but algorithms exist (Fig. 27.2). The patient should not have any definable contraindications to PNS. (The use of PNS may be possible in some patients who are not candidates for spinal cord stimulation because of comorbidities or increased risks.)
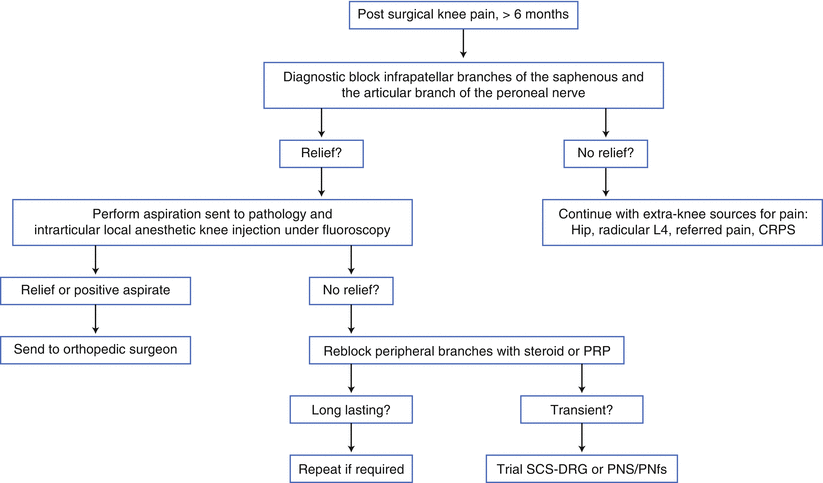

Fig. 27.2
Postsurgical knee pain work-up algorithm using a diagnostic nerve block. CRPS complex regional pain syndrome, PNfs peripheral nerve field stimulation, PRP platelet rich plasma
27.4 Technical Overview
The patient should undergo normal preoperative evaluation and testing. Attention should be given to hemostasis and infection risks. The integrity of the skin should be considered, and the absence of open lesions should be noted. Further, the presence of infectious postoperative knee complications should be fully interrogated and patient optimization should occur.
Once the patient has been deemed an appropriate candidate for the procedure, the use of preoperative antibiotics is recommended. The patient is brought to the operating theater after carefully mapping the nerve and pain distribution. If a conventional or new experimental trial is considered with PNS leads and no implantable programmable generator (IPG), attention is given only to the area of the limb having pain. The spacing, size, and number of leads should match the patient’s painful area appropriately.
In patients who have already undergone a trial, the planning of IPG placement is essential during this time of education and discussion. Common places are the medial or lateral thigh within the subcutaneous adipose tissue.
27.4.1 Lead Placement
In the early days of PNS implants, one of the authors (TRD) used the technique of lead placement on the diseased or targeted nerve. This laborious approach involved making a surgical dissection to the nerve and then placing a unidirectional paddle lead over the nerve. Prior to implanting the lead, the surgeon had to create a fascial graft to insulate the nerve. This approach led to an initial good outcome in most patients, but unfortunately, over time the nerve would become hypersensitive and the lead would become unusable. In modern PNS placement, advancements in programming have allowed a much less invasive approach. This method involves using a styletted needle to place a lead percutaneously close to the vicinity of the nerve, with fluoroscopic landmarks or ultrasound guidance. Once the lead is in place, intra-operative computer testing using various pulse-width, frequency, and electrode combinations are used to confirm appropriate nerve activation. The lead is sutured to the skin close to the entry point to enable evaluation of pain relief and functional improvement over a trial lasting 3–14 days.
27.4.2 Permanent Implant
A successful trial, characterized by a reduction in pain scores greater than 50 %, is usually followed by scheduling of the patient for a permanent implant. The lead placement for the permanent implant is performed in the same fashion as the trial. The lead position is determined by the hard-copy x-ray saved after trial placement. In some cases, the final lead placement may be modified based on feedback from the trial paresthesia. Once the lead is placed, hand-held programming is performed to document appropriate coverage. When the final lead position is determined, a cut-down is made in the area proximal to the electrodes. In this incision, the physician must use blunt dissection to identify fascia, which will be the point of anchoring for the suture to secure the lead. Anchoring can be performed with a nonabsorbable suture directly to the fascia and lead, or a commercial Silastic anchor can be used. If an anchor is used, the implanter must pay careful attention to the skin quality and tissue depth to ensure that adequate tissue covers the anchor, as the risk of an anchor is skin erosion. Once the lead is anchored, a strain relief loop is made with the lead, and the lead is tunneled to the pocket. Current devices require an IPG, so careful planning is needed to reduce the tunneling distance from the implant. Once the lead and generator are connected, a sterile hand-held programmer can be used to retest the paresthesia coverage and impedance. Impedance numbers less than 1500 are normally associated with acceptable tissue contact. When testing is concluded, the wound is vigorously irrigated and good hemostasis is established. At this time, a two-layer or three-layer closure should be performed and proper dressings placed (Figs. 27.3, 27.4, 27.5, and 27.6).