Peripheral and Central Sensitization Related to Headaches
Rami Burstein
Dan Levy
Moshe Jakubowski
Clifford J. Woolf
CLINICAL MANIFESTATIONS OF PERIPHERAL AND CENTRAL SENSITIZATION
Among the common symptoms of peripheral sensitization during migraine are the throbbing of the headache and its aggravation during routine physical activities such as coughing, sneezing, bending over, rapid head shake, holding one’s breath, climbing up the stairs, or walking (9,72). Such intracranial hypersensitivity involves the sensitization of nociceptors that innervate the meninges (89). Accordingly, fluctuations in intracranial pressure (24) associated with normal vascular pulsation (4 to 10 mmHg), as well as those associated with bending over or coughing (4 to 25 mmHg), effectively activate meningeal nociceptors during migraine (when they are sensitized), but not in the absence of migraine (when they are not sensitized).
Among the common symptoms of central sensitization during migraine is the phenomenon of allodynia, where patients become irritated by mundane mechanical and thermal stimulation of the scalp and facial skin (8,16,25,29, 40, 41, 42,54,55,81,93,98). This hypersensitivity is manifested in response to activities such as combing, shaving, breathing cold air, and wearing eyeglasses, contact lenses, earrings, or necklaces. Such allodynia involves the sensitization of nociceptive trigeminovascular neurons of the medullary dorsal horn that receive converging sensory input from the dura and skin (15). Accordingly, innocuous skin stimuli evoke dramatic activity in central trigeminovascular neurons during migraine (when they are sensitized), but produce little or no response in the absence of migraine (when they are not sensitized).
PERIPHERAL SENSITIZATION
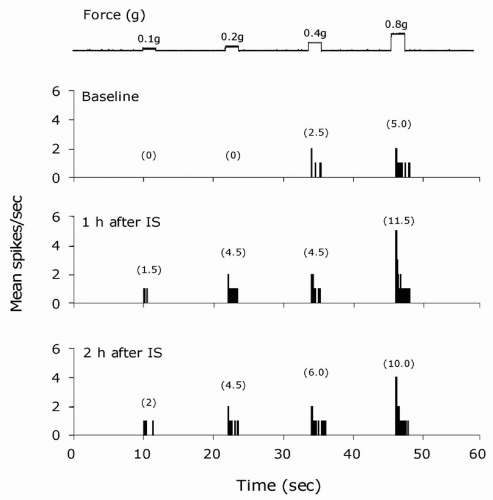
Peripheral sensitization is thought to be a major contributor to states of pain hypersensitivity in many painful syndromes including migraine headaches (2,50,89). It generally refers to a state where primary afferent nociceptive neurons exhibit increased responsiveness to external mechanical or thermal stimuli at the original site of inflammation or injury. Such changes can be manifested as a novel response to previously ineffective stimulus intensities, indicating decreased activation thresholds (22,52,61,79), and increased response magnitude (Fig. 12-1) to suprathreshold stimuli either with (32,90) or without (2,19) a noticeable change in threshold. In addition to marked changes in the stimulus response properties, peripheral sensitization can also be manifested as an increased level of ongoing discharge (i.e., spontaneous activity) in the absence of externally applied stimuli.
Inflammatory Mediators of Peripheral Sensitization
A large number of chemical mediators produced at the site of tissue injury and inflammation are capable of promoting the excitation and sensitization of nociceptors. Inflammatory mediators such as bradykinin, histamine, serotonin (5-HT), and prostaglandin E2 (PGE2), have been shown to produce both excitation and mechanical sensitization of somatic (87) and meningeal nociceptors (22,89). Other inflammatory mediators known to promote peripheral sensitization are pro-inflammatory cytokines, most notably interleukin (IL)-1, IL-6, and IL-8 and tumor necrosis factor-α (TNF-α) (56,77). These mediators are believed to promote nociceptor sensitization through the endogenous release of eicosanoids and sympathetic amines (77). Additional inflammatory mediators proposed to promote peripheral sensitization include protons, proteases, and nitric oxide. Increased levels of protons (acidic pH) found in inflamed tissues, produce not only activation and sensitization of meningeal nociceptors (89), but also enhances the effects of other inflammatory mediators (87).
Inflammatory proteases, especially tryptase and trypsins, activate protease-activated receptors (PARs) on nociceptors, most notably PAR-2 (33). Nitric oxide has been shown to produce local inflammation within the meninges (74), sensitize meningeal nociceptors (53), and induce headache or migraine in patients (68). Interestingly, release of nitric oxide from the endothelium can also be induced by bradykinin and histamine.
Inflammatory proteases, especially tryptase and trypsins, activate protease-activated receptors (PARs) on nociceptors, most notably PAR-2 (33). Nitric oxide has been shown to produce local inflammation within the meninges (74), sensitize meningeal nociceptors (53), and induce headache or migraine in patients (68). Interestingly, release of nitric oxide from the endothelium can also be induced by bradykinin and histamine.
Cellular Mechanisms of Peripheral Sensitization
Most sensitizing agents activate receptors that are coupled to second messenger cascades that, in turn, modulate voltage-gated ion channels. Other potential targets for the actions of sensitizing agents on nociceptors may also include direct action on sensory transduction elements. Mechanical and thermal sensitivity can be modulated independently in individual nociceptors, suggesting the existence of separate, possibly multiple, transduction mechanisms (5,70). For example, increased thermal skin sensitivity can be mediated by the transient receptor potential ion channel 1, a transducer of noxious heat (71,95) when its threshold is lowered by bradykinin, PAR-2 agonist, and protons (20,76,91). The mechanism underlying increased mechanical sensitivity remains largely unknown; transducers of noxious mechanical stimuli are yet to be identified.
Peripheral sensitization can also be promoted by changes in the properties of voltage-gated ion channels, such as the TTX-resistant (TTX-R) sodium channel. Inflammatory agents such as PGE2 and 5-HT are thought to sensitize sensory neurons by modulating TTX-R sodium currents (30) through activation of the cyclic adenosine monophosphate (cAMP)-PKA second messenger cascade (31). Such action is also likely to be involved in sensitization of mechanosensitive meningeal nociceptors as they express the TTX-R channels (88) and are sensitized by the cAMP-PKA cascade (52). The cAMP-PKA is also likely to be involved in mechanical sensitization through the suppression of the sustained (delayed rectifier) outward K+ current that is thought to modulate the firing threshold (27,28) and the enhancement of Ih, the hyperpolarizationactivated cation current that is thought to facilitate repetitive firing (39). Another second messenger cascade that may also promote mechanical sensitization is the cyclic guanosine monophosphate (cGMP)-PKG cascade that is activated by nitric oxide. As already noted, the sensitizing action of nitric oxide on meningeal nociceptors could also involve the activation of this cascade probably through the facilitation of Ca+2-activated potassium (BK) channels (47).
The proximate factors that cause local release of sensitizing chemicals during migraine remain unknown. One presumed factor is cortical spreading depression—a slowly propagating wave of neural inhibition (and excitation)
associated with extracellular release of excitatory agents such as potassium and glutamate. Bolay et al. (10) have recently shown that cortical spreading depression activates the trigeminovascular system. One of the potential consequences of sensory fiber activation is the release of neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP) from the peripheral terminals of meningeal nociceptors which, in turn, promotes vasodilatation and plasma extravasation (17,49,84). CGRP and SP are thought to sensitize nociceptors indirectly by inducing the release of sensitizing inflammatory mediators such as histamine, 5HT, BK, TNF-α, and nitric oxide from other immune cells, especially mast cells, in a process known as mast cell degranulation (75,92, 108).
associated with extracellular release of excitatory agents such as potassium and glutamate. Bolay et al. (10) have recently shown that cortical spreading depression activates the trigeminovascular system. One of the potential consequences of sensory fiber activation is the release of neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP) from the peripheral terminals of meningeal nociceptors which, in turn, promotes vasodilatation and plasma extravasation (17,49,84). CGRP and SP are thought to sensitize nociceptors indirectly by inducing the release of sensitizing inflammatory mediators such as histamine, 5HT, BK, TNF-α, and nitric oxide from other immune cells, especially mast cells, in a process known as mast cell degranulation (75,92, 108).
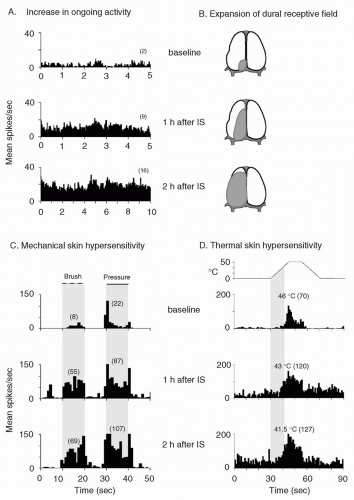 FIGURE 12-2. Central sensitization: physiologic characteristics of a sensitized central trigeminovascular neuron induced by topical application of inflammatory soup (IS) to the dura. A. Increased mean firing rate (from 2 to 16 spikes/sec) in the absence of external stimulus. B. Expansion of dural receptive field. C. Enhanced responses to mechanical skin stimulation (brush: from 8 to 69 spikes/sec; pressure: from 22 to 107 spikes/sec). D. Enhanced responses (from 70 to 127 spikes/sec) and decreased threshold (from 46 to 41.5°C) to thermal skin stimulation. Shaded areas depict receptive field in B; duration of mechanical stimulation in C; interval between onset of heating and response threshold at baseline in D. Numbers in parenthesis indicate mean spikes/sec. Adapted from reference 13. |
Most of other triggering factors are, however, likely to be extrinsic to the brain and may include increased stress level, change in hormone levels, and certain types of food. Such processes may directly or indirectly cause the release of excitatory and sensitizing molecules by promoting activation of inflammatory cells in the meninges, most notably mast cells (94), that are capable of releasing a large
number of excitatory and sensitizing mediators including histamine, nitric oxide, prostaglandins, and cytokines.
number of excitatory and sensitizing mediators including histamine, nitric oxide, prostaglandins, and cytokines.
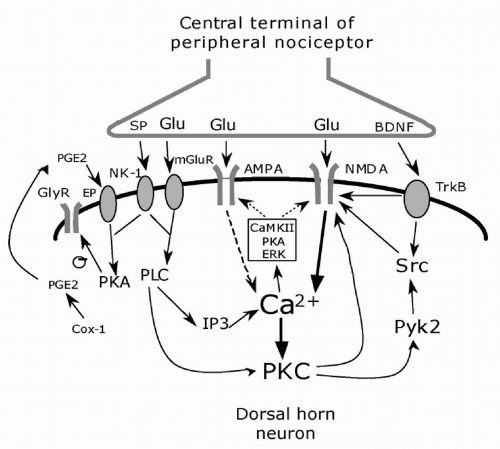 FIGURE 12-3. Cellular induction of central sensitization. The central terminals of peripheral nociceptor releases the neurotransmitter glutamate (Glu) and the neuromodulators substance P (SP) and BDNF. Glutamate binds to ionotropic AMPA and NMDA receptors and mGluR metabotropic receptors; SP and BDNF bind to the G-protein-coupled NK-1 receptor and tyrosine kinase receptor TrkB, respectively, on the postsynaptic membrane. An increase of intracellular Ca2+ is the major trigger for the activation of serine/threonine protein kinases, including PKA, PKC, and CaMKII. These kinases, as well as the tyrosine kinase Src, phosphorylate NMDA and AMPA receptors leading to increased sensitivity. In addition, ERK downstream to PKA and PKC, phosphorylates the Kv4.2 potassium channel. PKC induces rapid recruitment of AMPA receptors to the synapse. Adapted from reference 44. |
CENTRAL SENSITIZATION
Physiologic Properties
Central sensitization in somatosensory pain pathways was first discovered in the rat spinal cord, where it was shown to have a role in producing postinjury pain hypersensitivity (102), and later documented in several animal models and in humans (35,48,57,73,85,96). Central sensitization refers to a condition where nociceptive neurons in the dorsal horn of the spinal cord exhibit increased excitability, increased synaptic strength, and enlargement of their receptive fields beyond the original site of inflammation or injury (63,103,104). Central sensitization is triggered by sensory input arriving from sensitized nociceptors that supply the affected site. Once initiated, the sensitization of the central neurons may remain dependent on incoming input (activity dependent) or become self-sufficient altogether (activity independent). Sensitized dorsal horn nociceptors become responsive to innocuous (previously subthreshold) sensory signals that arrive from areas outside the affected site, resulting in expansion of their receptive fields. Accordingly, central sensitization is manifested clinically as decreased pain threshold and exaggerated pain response referred outside the original pain site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree