Objectives/indications
The presence of a pericardial effusion, pleural effusion, or ascites may warrant emergent drainage via pericardiocentesis, thoracentesis, or paracentesis, respectively. Though these are rare conditions in children, point-of-care ultrasound may assist in decreasing the complications associated with these procedures. The indications for pericardiocentesis, thoracentesis, and paracentesis are well summarized by the Society of Cardiovascular and Interventional Radiology Standards of Practice Committee as “the presence of an abnormal fluid collection with suspicion that the fluid is infected, the need for fluid characterization, or suspicion that the collection is producing clinically relevant symptoms.” Pericardiocentesis, thoracentesis, and paracentesis in children are especially complicated by their relatively small size and lack of cooperation compared to adults. Moreover, children often require procedural sedation in order to perform these procedures. The risks and benefits of procedural sedation should be considered carefully for each patient.
Clinically significant pericardial effusions are rare in children and can result from trauma, neoplasm, collagen vascular disease, or infection. A significant proportion of pericardial effusions in children are idiopathic. An abnormal fluid collection may develop in the pericardial sac either acutely or chronically. When fluid accumulation is acute, small pericardial effusions can result in tamponade. When fluid accumulation is chronic, the pericardium can actually accommodate large effusions without cardiovascular compromise. Tamponade occurs when pressure from the pericardial fluid impairs ventricular filling during diastole, which can compromise normal circulatory function and lead to the rapid deterioration of the patient. The classic clinical triad of hypotension, elevated systemic venous pressure, and muffled heart sounds is unreliable and lacks specificity. While relatively uncommon, when tamponade with cardiovascular compromise is present, an emergent aspiration of fluid from the pericardial sac, or pericardiocentesis, is indicated. Pericardiocenteses have been performed since the 1840s, but traditional “blind” approaches are associated with significant complications. Whether performed on an emergent or semi-elective basis in a controlled setting, ultrasound is recommended to identify pericardial effusions, and to guide pericardiocentesis.
In contrast, pleural effusions are not uncommon in pediatric patients. The most common causes of pleural effusions in otherwise healthy children are pulmonary infections such as parapneumonic effusions. Other causes of pleural effusions include congestive heart failure, malignancy, and renal disease. Point-of-care ultrasound is a valuable tool in the diagnosis and characterization of these effusions. If an abnormal fluid collection is identified in the pleural space (by ultrasound or by other imaging modalities), ultrasound may be used to assist in performing thoracentesis. The two main indications for thoracentesis are (1) to obtain pleural fluid for diagnostic purposes and (2) to drain pleural fluid that is compromising normal respiratory function. When thoracentesis is performed for diagnostic purposes, it is generally performed on a semi-elective basis in a controlled setting, such as the operating room. However, thoracentesis may need to be performed more emergently, when it is required to alleviate respiratory compromise.
Finally, ascites is a rare condition in pediatrics. Causes of ascites in children include hepatocellular disease, congestive heart failure, cardiac abnormalities, infection, pancreatic disease, and low albumin states. While reasonably safe in adults, paracentesis is a relatively uncommon procedure performed in pediatric patients. However, paracentesis may be indicated to aid in the diagnostic evaluation of patients who present with ascites, and especially when bacterial peritonitis is suspected. Paracentesis may also be performed to alleviate symptoms caused by ascites, such as pain or respiratory distress.
Although pediatric-specific literature is scarce, using point-of-care ultrasound to assist in pericardiocentesis, thoracentesis, and paracentesis may increase the safety and likelihood for procedural success. The principles of ultrasound assistance for these procedures are similar for pediatric patients and adults. However, adjustments may need to be made for children because of their relatively small size. For each procedure, ultrasound may be used to mark the optimal incision site (ultrasound assistance) or to actively guide needle insertion (ultrasound guidance). When using real-time ultrasound guidance, novice users should strongly consider using a dual-operator approach, with one person operating the ultrasound device and the other performing the procedure.
Pericardiocentesis
Background
Conventionally, pericardiocentesis that is performed by the non-cardiologist is performed using the landmark technique, with a subxiphoid approach. Complications of this technique are common and include myocardial injury, coronary artery laceration, pneumothorax, dysrhythmias, injury to the diaphragm and subdiaphragmatic organs, and even death. Most of the literature supporting ultrasound assistance and ultrasound guidance for pericardiocentesis derives from the interventional cardiology literature, where pericardiocentesis is performed in a controlled environment, on a semi-elective basis. In this setting, echocardiographically guided pericardiocentesis has been used to identify the optimal site for needle placement, and has been shown to increase success rates and reduce complications of the procedure. Tsang et al. studied 1127 ultrasound-guided pericardiocenteses and demonstrated an overall success rate of 97%, with a complication rate of 4.7% (major 1.2%; minor 3.5%). In addition, echocardiographically guided pericardiocentesis has also been found to be safe in pediatric patients. As a result, echocardiographic guidance has become standard for pericardiocentesis in cardiology.
While the principles of echocardiographically guided pericardiocentesis can be extrapolated to the pediatric intensive care and emergency department settings, more research is needed to support the routine use of point-of-care ultrasound to assist in pericardiocentesis in these settings. Based on the established literature thus far, the American Society of Echocardiography Consensus Statement specifically recommends that, when emergent pericardiocentesis is indicated in the emergency department, an ultrasound first be performed in order to determine the best needle trajectory, followed by ultrasound-guided pericardiocentesis. In hemodynamically unstable patients with pericardial effusions, a comprehensive echocardiogram need not be performed.
Anatomy
The anatomy of the heart is discussed in Chapter 5 (Figure 5.1). The heart is surrounded by the inner visceral and outer parietal pericardium. Normally, a trace amount of physiologic fluid provides lubrication between these two layers, in the pericardial sac. A pericardial effusion develops between these two layers.
Technique
Transducer selection and orientation
A low-frequency, phased-array transducer should be used to obtain the standard cardiac views (see Chapter 5). The parasternal long-axis or apical views are preferred when performing ultrasound-guided pericardiocentesis, since they provide a more direct anatomic approach. In the parasternal long-axis view, the indicator is oriented towards the patient’s left hip (Figure 21.1a). Alternatively, in the subxiphoid view, the transducer is flattened, below the xiphoid process, with the indicator oriented towards the patient’s right side (Figure 21.2a).
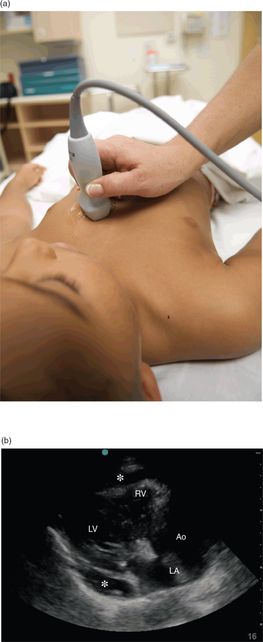
Figure 21.1 The evaluation of a pericardial effusion, parasternal long-axis view. (a) Transducer placement. (b) Ultrasound image of a pericardial effusion (*). Note the effusion appears as an anechoic stripe between the two layers of the pericardium. This effusion is loculated and does not extend circumferentially around the heart. Other structures seen are the left ventricle (LV), right ventricle (RV), left atrium (LA), and aorta (Ao).

Figure 21.2 The evaluation for a pericardial effusion, subxiphoid view. (a) Transducer placement. (b) Ultrasound image of a pericardial effusion (*). Other structures visualized are the right atrium (RA), right ventricle (RV), left atrium (LA), and left ventricle (LV). Ultrasound image courtesy of Jason Levy, MD RDMS.
Patient position and preparation
If clinically feasible, the patient should be positioned in an upright, seated, or a left-lateral decubitus position. When performing pericardiocentesis, sterile preparation of the patient and transducer should be carried out whenever possible.
Ultrasound imaging
Both the parasternal long-axis (Figure 21.1b) and the subxiphoid (Figure 21.2b) views can confirm the presence of a pericardial effusion, can delineate the extent of the effusion, and can identify the relationship of the fluid to the chest wall. Pericardial fluid will appear as an anechoic or hypoechoic stripe between the two layers of the pericardium. When tamponade is clinically suspected, the sonographic findings of right ventricular diastolic collapse, a circumferential pericardial effusion, and hyperdynamic heart support the diagnosis. Because physical examination findings for tamponade are unreliable, point-of-care ultrasound can be used to rapidly confirm the presence of a pericardial effusion and assess its hemodynamic effects prior to performing pericardiocentesis.
Ultrasound-guided procedure
When using ultrasound, the optimal needle placement for pericardiocentesis is the location where the effusion is closest to the transducer and the fluid collection is the greatest. For the static technique, the angle of interrogation of the transducer and depth from the skin to the pericardial fluid should be noted and used to determine the angle and the depth of needle insertion. This site can be marked on the patient’s skin prior to the procedure.
Alternatively, if the patient’s clinical status permits, ultrasound guidance may be utilized, in which real-time imaging visualizes the needle insertion and advancement (Figure 21.3). This offers the advantage of the direct visualization of the needle and the ability to redirect the needle’s approach in real time, and decreases the uncertainty about the accuracy of the needle’s position within the pericardial space. With ultrasound guidance, the parasternal long-axis approach is preferred, since it involves a more direct anatomic approach. This is in contrast to the blind methods, which traditionally utilize a subxiphoid approach, and involve a needle trajectory directly through the liver. The injection of agitated saline may help localize the needle during the ultrasound-guided procedure.
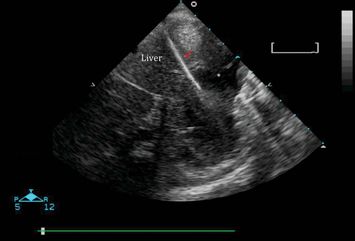
Figure 21.3 Pericardiocentesis. Real-time ultrasound guidance shows the needle (arrow) entering the pericardial space to drain a pericardial effusion (*). Image courtesy of Mahmoud Elbarbarry, MD MSc PhD.
Training and education
When training practitioners to perform ultrasound-assisted and ultrasound-guided pericardiocentesis, it is important to first become proficient in performing cardiac ultrasound and obtaining the different views of the heart (see Chapter 5). Commercially available simulators and phantom models may be utilized to demonstrate pericardial effusions, with the ability to practice the procedure. Recently, Zerth et al. described a low-cost, easily constructed task trainer to simulate ultrasound-guided pericardiocentesis (Figure 21.4).
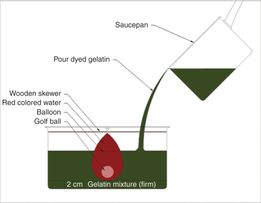
Figure 21.4 Homemade model for pericardiocentesis. Low-cost materials include a gelatin mixture, colored water, golf ball, balloon, and wooden skewer. Source (copyright): Zerth, H., Harwood, R., Tommasso, L., Girzadas, D. V. Jr. (2011) An inexpensive, easily constructed, reusable task trainer simulating ultrasound-guided pericardiocentesis. J Emerg Med 43(6): 1066–9.
Thoracentesis
Background
Historically, thoracentesis has been performed using a landmark technique in conjunction with physical examination and X-ray findings. Complications of thoracentesis include pneumothorax, hemothorax, lung laceration, hepatic laceration, and splenic laceration. Failures, or “dry taps,” are not uncommon.
Several studies have shown an advantage to ultrasound-assisted thoracentesis in adult patients. The use of ultrasound to mark the needle insertion site immediately prior to performing thoracentesis has been shown to reduce the rate of pneumothorax and to increase the accuracy of the insertion site. The risk of an iatrogenic pneumothorax after ultrasound-guided thoracentesis is 0 to 2.5%, in contrast to blind approaches, where the risk ranges from 4 to 30%. Ultrasound has also been used to assist successful thoracentesis following failed attempts by traditional techniques. It is especially useful in patients in whom a thoracentesis is expected to be difficult, such as patients with small or loculated effusions, and mechanically ventilated patients.
While ultrasound has been used to identify pleural effusions in children, there are no known pediatric-specific studies comparing ultrasound-assisted to traditional thoracentesis. Further, it is extremely rare to require emergent thoracentesis in children; the procedure is most often performed in the controlled operating room setting.
Anatomy
As discussed in Chapter 6, the lung is surrounded by two layers of pleura: the inner visceral pleura and the more superficial parietal pleura. A trace amount of physiologic fluid provides lubrication between these two layers during normal respiration. Abnormal fluid can collect in the potential space between the parietal and visceral pleura as a result of infection, inflammation, congestive heart failure, or anatomic abnormalities. Pleural fluid initially collects in the potential space known as the costodiaphragmatic recess. In an upright or prone patient, pleural fluid will first accumulate in the most dependent parts of the pleural space, posteriorly and inferiorly.
Technique
Transducer selection and orientation
The selection of the appropriate transducer depends on the patient’s size and the operator’s preference. In adults, evaluation of the pleural space is often performed with a curvilinear, low-frequency (2–5 MHz) transducer. However, in children, the smaller footprint of the phased-array transducer may provide better views through the relatively small intercostal spaces. Alternatively, a high-frequency, linear transducer may be utilized when less penetration is needed. The transducer is positioned with the indicator towards the patient’s right or the patient’s head.
Patient position and preparation
To evaluate the presence of a pleural effusion, the patient should be seated upright whenever possible (Figure 21.5a). In order to improve image acquisition and patient comfort, the patient can lean slightly forward over a bedside table or chair for support, with his/her arms positioned forward or over the head. In patients who cannot be seated upright, such as mechanically ventilated patients, ultrasound may be performed with the patient prone or in the lateral decubitus position (affected side up) with the ipsilateral arm brought across the chest.
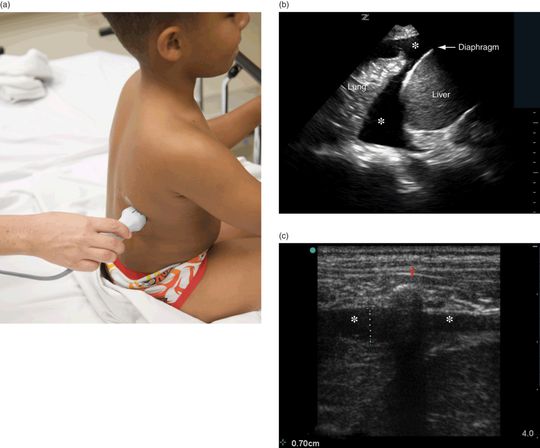
Figure 21.5 The evaluation of pleural effusions; preparation for thoracentesis. (a) Transducer placement. (b) Ultrasound image of a pleural effusion (*) with the phased-array transducer. Surrounding structures include the liver, lung, and diaphragm. (c) Ultrasound image of a pleural effusion (*) with the linear transducer. A rib and its posterior shadow (red arrow) can be seen and aid in the identification of the pleural line. A pleural effusion (*) appears as a hypoechoic or an anechoic collection between the two pleural layers. Ultrasound images courtesy of James Hwang, MD RDMS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








