Pericardial Diseases
Carlos A. Roldan
The history and physical examination are essential in the diagnosis of acute pericarditis, cardiac tamponade, and constrictive pericarditis. However, with the exception of pericardial rub and pulsus paradoxus, symptoms and physical findings in pericardial diseases are not specific and are highly variable.
Cardiac tamponade can be diagnosed clinically, but frequently patients with hemodynamic evidence of tamponade may not have typical physical findings (Table 17.1).
Similarly, constrictive pericarditis cannot be differentiated clinically from other causes of diastolic or systolic heart failure.
Class I or Appropriate (Score 7–9) Indications for Echocardiography in Pericardial Diseases
Echocardiography (echo) is needed to determine the presence and size of a pericardial effusion; pericardial thickening, calcification, or masses; and to assess hemodynamic findings suggestive of cardiac tamponade or constriction (1,2) (Table 17.2).
Table 17.1 Physical findings in patients with cardiac tamponade
Physical Finding
Frequency (%)
Elevated jugular venous pressure
40–100
Sinus tachycardia
50–75
Pulsus paradoxus (≥20 mm Hg)
17–75
Hepatomegaly
25–55
Distant heart sounds
25–35
Systolic blood pressure <100 mm Hg
15–35
Pericardial rub
25–30
Peripheral edema
20–30
The lowest rates are found in patients with only echocardiographic findings suggestive of cardiac tamponade or in those with atypical forms of tamponade (i.e., post cardiac surgery). The highest rates are found in those with clinical evidence of tamponade.
Echo plays an important role in the inpatient or outpatient diagnosis and management of acute
pericarditis and its associated complications; in the safe performance of an urgent or elective therapeutic or diagnostic pericardiocentesis in a patient with cardiac tamponade or a moderate to large pericardial effusion without tamponade, respectively; and in the decision to perform cardiac catheterization or surgery, or both, in a patient with constrictive pericarditis (3,4,5).
Table 17.2 Class I or appropriate (score 7–9) indications for echocardiography in patients with pericardial diseases
Symptoms potentially related to pericardial disease such as pleuritic chest pain or shortness of breath.
Abnormal electrocardiogram, chest radiography, or cardiac biomarkers concerning for pericardial or myopericardial disease.
Hypotension or hemodynamic instability of suspected pericardial etiology.
In patients with suspected pericarditis, pericardial effusion, tamponade, constriction, or effusive-constrictictive pericarditis.
Re-evaluation of known pericardial effusion to guide management or assess response to therapy.
Follow-up study to evaluate recurrence of effusion, tamponade, or early constriction.
In patients with acute myocardial infarction and persistent pain, hypotension, or a friction rub.
In patients after heart surgery with persistent hypotension to exclude pericardial hematoma or atypical cardiac tamponade.
Follow-up study to detect early signs of tamponade when large or rapidly accumulating effusions are seen.
Penetrating or non-penetrating chest trauma when pericardial effusion is possible or suspected.
Echocardiographic guidance and monitoring of pericardiocentesis.
Evaluation of pericardial window procedures in patients with posterior or loculated pericardial effusions (intraoperative).
(Adapted from Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166).
Echo has special diagnostic and therapeutic value in patients with localized cardiac tamponade in whom clinical and hemodynamic findings are commonly atypical or absent.
Acute Pericarditis Without or with Pericardial Effusion
Definition
Acute pericarditis is characterized by inflammation of the parietal and visceral pericardium and associated with either no or small to moderate (rarely large) serous, serosanguineous, serofibrinous, hemorrhagic, chylous, cholesterol-laden, or purulent pericardial effusion.
Pericardial thickening, fibrosis, fusion, and, uncommonly, calcification can occur.
Associated myocarditis (based on myocardial isoenzymes elevation, wall motion abnormalities, or decreased ejection fraction) is seen in at least 15% of cases (6).
Common Etiologies
Infection, autoimmunity, malignant metastatic infiltration, surgical or nonsurgical trauma, mediastinal radiotherapy, and vasculitidis are the most common causes (3,6).
In the general population of developed countries, viral infection (including HIV), idiopathic, and post myocardial infarction or post cardiotomy syndrome are the most prevalent causes (2,3,5,6).
In developing countries, bacterial, mycobacterial, and parasitic infections are common.
In bacterial pericarditis, pneumococcus, streptococcus, and staphylococcus are the most common microorganisms, and they more often infect the pericardium by contiguous extension from the lung, pleura, or mediastinum and rarely by hematogenous spread.
Its mortality ranges from 30% to 50% and is highest in patients with cardiac tamponade (7).
Pericardial Morphology on M-Mode and Two-dimensional Echocardiography
Best Imaging Planes
Definitions and Key Diagnostic Features
Pericardial effusion is a separation of the visceral and parietal pericardium by fluid (>30 mL) and is associated with a decrease motion of the parietal pericardium.
Pericardial thickening is present when either the parietal or visceral or both pericardial layers measure ≥3 mm.
A pericardial effusion, especially if associated with pericardial thickening or fusion, confirms pericarditis in a patient with a consistent clinical syndrome (Fig. 17.1).
Absence of a pericardial effusion does not exclude pericarditis, however, because an effusion is not seen in >30% of patients with clinical pericarditis.
Also, pericardial effusion does not establish the diagnosis of pericarditis. Patients with nephrosis, malnutrition, and heart failure (more commonly with right heart failure) have small pericardial effusions without pericarditis.
Characteristics of Pericardial Effusions
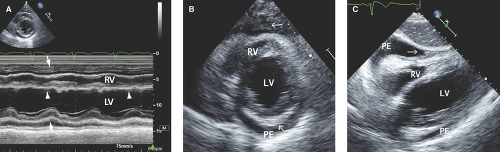
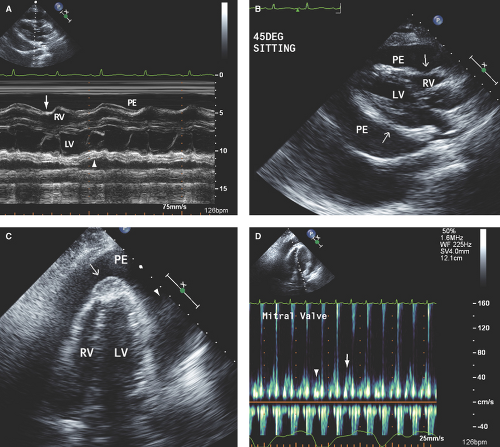
Small pericardial effusion (<100 mL) is seen only posterior to the left ventricle (LV), distal to the atrioventricular groove, and with <1 cm separation of the pericardial layers (Fig. 17.1).
Moderate pericardial effusion (100 to 500 mL) is seen when fluid accumulation is circumferential, extends posterior to the left atrium (LA), and the separation of pericardial layers is still <1 cm.
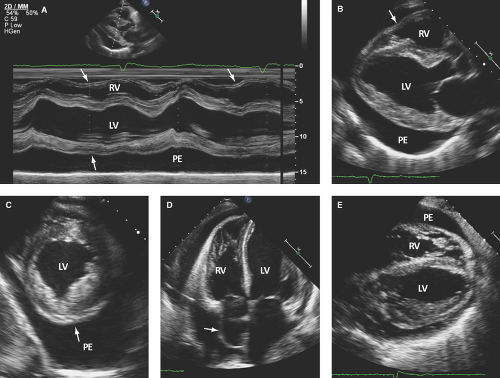
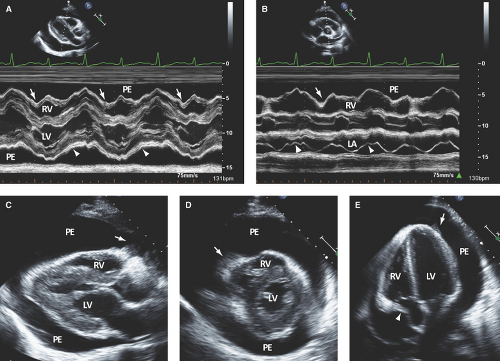
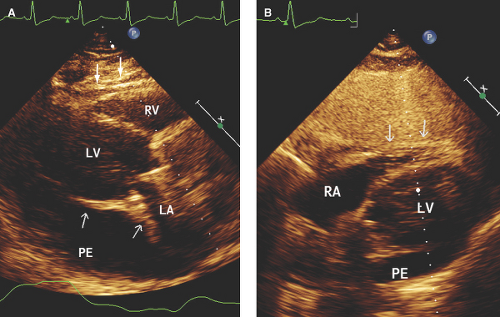
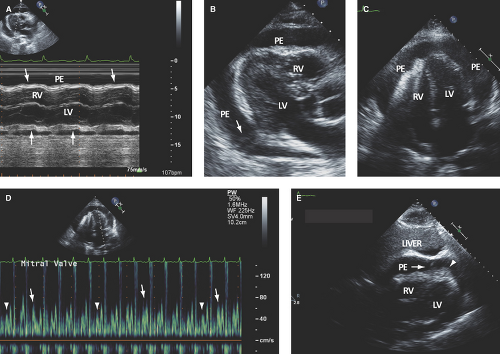
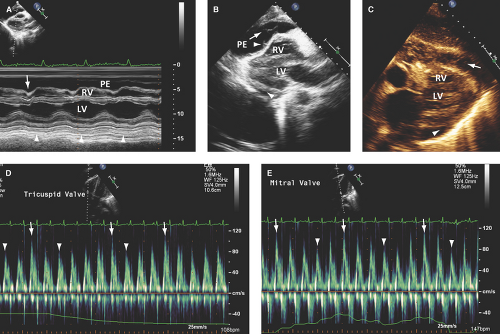
Large pericardial effusion (>500 mL) is seen when there is circumferential fluid accumulation, >1 cm separation of pericardial layers, and anteroposterior or mediolateral heart swinging within the pericardial sac (Figs. 17.2 to 17.6).
Loculated effusions are uncommon in patients with clinical pericarditis. When present, they usually are single,
of variable size and location (more common posterolaterally to the LV), and fluid is surrounded by thickened and fused pericardium. These effusions are common post cardiac surgery (more common after prosthetic valve replacement because of the need for anticoagulation) (Fig. 17.1C).
Echogenic pericardial effusions are seen in about 20% of patients with moderate to large pericardial effusions and are caused by inflammatory fronds, strands, or diffuse echogenicity (8). Uremia and heart failure are common causes of nonechogenic pericardial effusions:
Intrapericardial fronds and strands are aggregates of fibrin, thrombus, or rarely tumor and are indicative of noninfective or infective inflammation.
Fronds appear as soft tissue echoreflectant sessile masses of variable size, most commonly seen on the visceral pericardium (Figs. 17.3C–E and 17.5C).
Strands appear as heterogeneously echoreflectant linear, undulated, or hypermobile structures, which can be single, multiple, or weblike and usually extend from the visceral to parietal pericardium (Figs. 17.6B,E and 17.7B,C).
Echogenic pericardial effusions are associated with a 20% to 30% incidence of recurrent pericardial effusions or constrictive pericarditis.
Differential Diagnosis of Pericardial Effusion
Epicardial fat has a speckled or granular echoreflectance with commonly associated hyperreflectant septations; is seen anterior, anteroapical, and posterolateral to the right ventricle (RV) and rarely posterior to the LV; and is more prevalent in the elderly, obese, diabetic, and women (<1% in those <30 years old and up to 15% in those >80 years old) (9). Thus, an anterior echo-free or speckled echoreflectant space is usually epicardial fat rather than a loculated pericardial effusion.
A left pleural effusion extends behind the descending aorta, in contrast to a posterior pericardial effusion, which extends anterior to the descending aorta and posterior to the LA (Fig. 17.5B).
Pitfalls of M-Mode and Two-dimensional Echocardiography in Acute Pericarditis
Pericardial effusion and pericardial thickening are frequently absent.
Low sensitivity for detecting pericardial thickening (40% to 60%) or nodules or masses (<40%) (5).
Low sensitivity for detection of loculated pericardial effusions in patients after cardiac surgery.
Although able to detect echogenic pericardial effusions, the type of pericardial fluid (blood, pus, or tumor) cannot be differentiated.
Cardiac Tamponade
Definition
Pericardial effusion leading to an increased intrapericardial pressure (>3 mm Hg);
Cardiac compression resulting in decreased filling of right and left heart chambers;
Increased and equalization of intrapericardial, right heart, pulmonary artery diastolic, and pulmonary capillary wedge pressures; and
Decreased cardiac output and blood pressure and increased heart rate and systemic vascular resistance.
Common Etiologies
Cancer (breast, lung, melanoma, or lymphoma; 30% to 60% of cases), uremia (10% to 15% of cases), acute or chronic idiopathic pericarditis (5% to 15% of cases), infection (5% to 10% of cases), anticoagulation (5% to 10% of cases), connective tissue diseases (2% to 6% of cases), and Dressler or postpericardiotomy syndromes (1% to 2% of cases) (2,4,9).
Heart surgery (bypass grafting and/or valve repair or replacement) and percutaneous coronary interventions, such as mitral balloon valvuloplasty, atrial septal defect closures, septal ablation, ablation of
atrial or ventricular arrhythmias, and valve replacement have all been associated with cardiac tamponade (10,11,12,13).
Pathophysiology
Normally, with inspiration, there is an increase in negative intrathoracic and intrapleural pressures leading to a decrease of >5 mm Hg in the intrapericardial and right heart pressures, resulting in an increased venous return. A simultaneously decreased pressure in the pulmonary veins results in a decreased filling of the left heart.
In cardiac tamponade and during inspiration, a mild decrease in the intrapericardial and right heart pressures results in increased RV filling and a consequent leftward interventricular septal displacement that limits LV filling. A simultaneously decreased pressure in the pulmonary veins results in further decrease of LV filling.
The end result is a low stroke volume and cardiac output, low blood pressure, and low pulsed volume during inspiration (pulsus paradoxus).
Echocardiography
The onset and severity of echo manifestations in cardiac tamponade vary according to the following:
Rate, severity, and extent (global or localized) of fluid accumulation.
Patient’s intravascular volume status (low-pressure tamponade in volume depletion).
Associated myocardial disease (absent chamber compression and pulsus paradoxus if high RV or LV end-diastolic pressure).
Underlying pericardial disease (effusive-constriction if pericardial thickening or fibrosis is present).
Clinically evident cardiac tamponade is highly predictive of echo findings of tamponade. In contrast, in patients without clinical cardiac tamponade, echo identifies those with none to mild hemodynamic compromise. However, this assumes a careful physical examination.
Patients with loculated pericardial effusions or hematomas have atypical clinical, echo, or hemodynamic findings of tamponade. In these patients, echo has high diagnostic value.
Thus, cardiac tamponade is a clinical syndrome with a spectrum of severity rather than an all or none phenomenon, and an accurate diagnosis requires integration of clinical, echo, and hemodynamic data.
M-Mode and Two-dimensional Echocardiography
Best Imaging Planes
M-mode TTE parasternal long- or short-axis and subcostal views.
Two-dimensional TTE parasternal long- and short-axis views and apical and subcostal four-chamber views.
TEE transgastric short- and long-axis and midesophageal four-chamber views.
Key Diagnostic Features
M-mode defines better than 2D echo the presence, timing, and severity of RV, LV, and LA diastolic compression. Both RV and LV compression occur during early diastole and resolve in late diastole (after atrial kick) and right atrium (RA) or LA compression occur during late diastole (Figs. 17.2A, 17.3A,B, 17.5A, 17.6A, and 17.7A).
Table 17.3 Characteristics of right heart diastolic compression in cardiac tamponade
Right Atrium
Right Ventricle
Occurs when IPP is ≥4 mm Hg
Occurs when IPP is ≥6–8 mm Hg
Most common and earliest finding
Occurs after right atrial compression
High sensitivity but low specificity and positive predictive value
Lower sensitivity than right atrial compression but higher specificity and positive and negative predictive values
Occurs during late diastole/early systole and is worse during expiration or apnea
Occurs during early diastole; may be transient or lasting throughout early and mid-diastole and disappear after atrial contraction
Duration of one-third or more of cardiac cycle is a better predictor of tamponade
Degree and duration of right ventricular collapse do not correlate with severity of cardiac tamponade
Best noted on midportion of right atrial lateral wall
Best noted on right ventricular anterior and posterolateral walls and infundibulum
Best seen from apical and subcostal views
Best seen from parasternal long- and short-axis views and subcostal view
IPP, intrapericardial pressure.
By M-mode, decreased stroke volume during inspiration results in a decreased mitral valve opening and E-F slope (Figs. 17.3A and 17.5A). M-mode may also identify pericardial thickening (Figs. 17.1A, 17.3A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree