(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
Goal directed therapySurgical outcomesCardiac outputFluid responsivenessCentral venous oxygen saturationEsophageal DopplerBioreactancePulmonary arty catheterMore than 230 million major surgical procedures are undertaken worldwide each year [1]. Data from the USA and Europe suggests that approximately 18 % of patients undergoing surgery will develop a major postoperative complication and 3–5 % will die before hospital discharge [1–4]. If this rate is applicable worldwide approximately 40 million patients undergoing surgery each year will die or develop a major postoperative complication. Those patients who develop a postoperative complication and survive to hospital discharge have diminished functional independence and reduced long-term survival. In a landmark study Khuri and coworkers demonstrated that survival up to 8 years after major surgery was strongly related to the development within 30 days of surgery of a major postoperative complication [4]. In this study, independent of preoperative risk, the occurrence of a 30-day complication reduced median patient survival by 69 %. In both the USA and Europe there are large variations in postoperative morbidity and mortality within healthcare systems [2, 3, 5]. Interventions that reduce the risks of postoperative death and complications, particularly in high risk patients have become a priority in perioperative medicine [6]. Interventions such as the use of perioperative beta-blockers, statins and tight glycemic control have proved disappointing [7, 8]. Pre-emptive goal-directed hemodynamic therapy (GDT) appears to be a promising approach to reduce postoperative complications and deaths.
Due to the assumed intravascular deficit after fasting, the increased insensible losses with damage to the skin barrier and the large shift of fluid into the “third space” generations of anesthesiologists have infused large volumes of fluids into patients. This approach resulted in pre-operative fluid loading and the intraoperative infusion of fluid at rates of up to 20 mL/kg/h (independent of blood loss). Traditional fluid management during major surgery resulted in a positive fluid balance of several liters and a perioperative body weight gain of up to 10 kg [9]. However, the insensible perspiration and the preoperative deficits are in fact often negligible, and the “third space” appears to be myth that does not exist [9]. The excessive interstitial edema that accumulates postoperatively is likely a consequence of excessive fluid administration. Indeed, multiple studies have demonstrated that a conservative fluid strategy as compared to “usual fluid management” is associated with fewer postoperative complications [10]. In a multicentre study, Brandstrup and co-workers randomized patients undergoing major colorectal surgery to a restrictive fluid strategy or usual care [11]. The patients in the restrictive group received less fluid (mean 2,740 vs. 5,388 mL) and had a significantly reduced incidence of major and minor complications. However, an overly restrictive fluid strategy will decrease cardiac output and oxygen delivery and likely increase complications. Futier et al. compared a restrictive to a conservative fluid strategy in patients undergoing major abdominal surgery. In this study the complication rate was significantly higher in the restrictive group. Goal directed therapy (GDT) provides a balance between the liberal and restrictive fluid strategies. GDT is a conservative approach to fluid management that is based on optimizing each individual patient’s hemodynamic profile. This approach represents a refinement of the “supranormal” hemodynamic approach pioneered by William Shoemaker in the 1980s [12].
In general GDT is based on the titration of fluids and inotropic drugs to physiological flow-related end points. Over 30 GDT studies have been performed to date and an analysis these studies suggest that peri-operative optimization of hemodynamics reduces postoperative complications in elective non-cardiac surgical patients [13–17]. In addition, GDT has been demonstrated to reduce the risk of death in high-risk patients [13–17]. On the basis of these data, the UK National Health Service’s National Institute for Health and Clinical Excellence (NICE) has recommended GDT (using esophageal Doppler) for patients undergoing major or high-risk surgery [18, 19]. This guidance states that “there is a reduction in postoperative complications… and in–hospital stay when compared with conventional clinical assessment with or without invasive cardiovascular monitoring… with an average cost saving of approximately £1,100 (about $1,800) per patient” [18, 19]. Despite these recommendations hemodynamic optimization during major surgery has not been widely adopted [20].
A number of different technologies (pulmonary artery catheter, esophageal Doppler and pulse contour analysis, bioreactance) and treatment algorithms have been utilized to optimize peri-operative hemodynamics. Most of these studies have used both fluids and inotropic agents to optimize cardiac output. Furthermore, GDT has been initiated pre-operatively, intra-operatively or postoperatively. Most of these studies have compared GDT to standard care (liberal fluid strategy) and have demonstrated that this approach improves outcomes in elective non-cardiac surgical patients [13–17]. Few “head-to-head” studies have been performed comparing different GDT strategies, therefore the optimal approach remains unclear [21–23]. The initial preemptive hemodynamic studies used the pulmonary artery catheter and targeted the Shoemaker “supranormal” goals while more recent studies have “optimized” cardiac output using esophageal Doppler, dynamic indices of fluid responsiveness or bioreactance.
It is widely believed that an oxygen debt occurs intraoperatively and that this oxygen debt increases the risk of postoperative infectious complications, wound breakdown, myocardial ischemia, acute kidney injury and death [13–17]. Furthermore, it is postulated that GDT with the optimization of intraoperative cardiac output prevents this intra-operative oxygen debt [13–17]. Figure 11.1 is a meta-analysis of GDT studies stratified by the timing of the initiation of hemodynamic optimization. This meta-analysis together with previous meta-analyses demonstrates that hemodynamic optimization improves outcome whether this intervention be initiated pre-operatively, intra-operatively or postoperatively [13–17, 24–26]. This observation casts doubt on the intraoperative oxygen debt theory. Furthermore, a critical analysis of these studies suggests that tissue dysoxia occurs postoperatively rather than intraoperatively (discussed further below). These observations have important implications for the management of surgical patients and suggest that the immediate postoperative management of high risk surgical patients may play a pivotal role in reducing postoperative complications and death. This is an important issue as the vast majority of patients who die are not admitted to an ICU after surgery. In the European Surgical Outcomes Study (EuSOS) 73 % of patients who died were not admitted to an ICU at any stage after surgery [2]. In a study of 26,051 patients undergoing non-cardiac surgical procedures performed in a large National Health System Trust only 35.3 % of high-risk patients were admitted to a critical care unit after surgery [27].
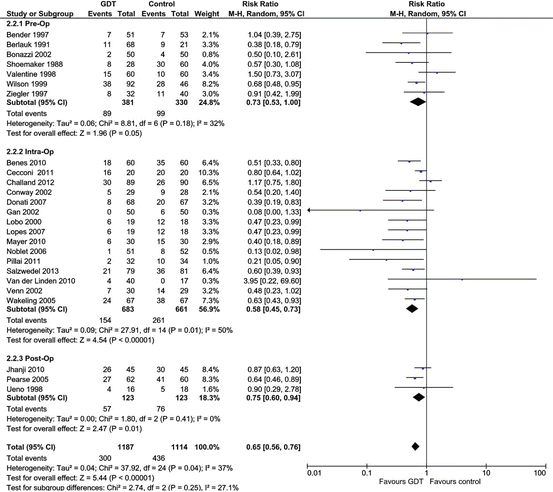

Fig. 11.1
Comparison of the risk of postoperative complications in studies that compared GDT (Protocol) versus standard therapy (control). Studies are grouped by timing of the initiation of hemodynamic optimization. Weight is the relative contribution of each study to the overall treatment effect (risk ratio and 95 % confidence interval) on a log scale assuming a random effects model. Metaanalysis performed using Review Manager 5.1
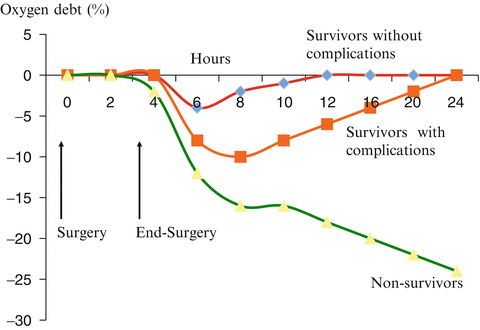
In 1988 Shoemaker and colleagues published a now landmark study in which they measured oxygen delivery (DO2) and oxygen consumption (VO2) in 100 consecutive patients undergoing high-risk surgical operations [28]. They calculated the intraoperative and postoperative oxygen debt (VO2 debt) by subtracting the measured VO2 from the estimated VO2 requirements corrected for temperature and anesthesia. It should be noted that the estimated VO2 during anesthesia was calculated using a non-validated equation [28]. These authors then correlated the calculated VO2 deficit with the subsequent development of lethal and non-lethal organ failure. In this study the cumulative VO2 deficit averaged 33.5 ± 36.9 L/M2 in non-survivors, 26.8 ± 32.1 L/M2 in survivors with organ failure and 8.0 ± 10.9 L/M2 in survivors without organ failure (p < 0.05). Shoemaker and colleagues noted that the oxygen debt was incurred almost exclusively during the intraoperative period. Based on these findings the authors proposed that the greater the oxygen debt incurred (during surgery) the greater the risk of organ failure and death [28]. Subsequently, these authors performed a number of pseudo-randomized studies in which patients were “randomized” to achieve supranormal hemodynamic goals (DO2 600 mL/min/m2 and CI > 4.5 L/min/m2) or standard care [25, 26]. In these studies the risk of complications and death were significantly lower in the “supra-normal” group. Based on the VO2 debt incurred intraoperatively and the summation of their outcome data Shoemaker and colleagues recommended that “in the high-risk patient, PA catheterization should be instituted preoperatively and that the important cardiorespiratory values be prophylactically augmented beginning in the preoperative and continued into the intraoperative and immediate postoperative periods” [26, 28].
In their 1988 paper Shoemaker and colleagues were unable to determine “which of these influences are operative” to explain the intraoperative oxygen debt [28]. Furthermore, as already stated the VO2 deficit was calculated using a formula that had not been validated [29]. At face value it would appear to be counterintuitive that anesthesia would result in an oxygen debt. General anesthesia and neuromuscular blockade reduce metabolic rate and oxygen consumption while DO2 remains largely unchanged [30, 31]. Hypothermia occurs frequently during anesthesia which further reduces metabolic oxygen requirements [32, 33]. Indeed, in Shoemaker’s pivotal paper VO2 fell during the intraoperative period reaching a nadir and the end of surgery [28]. In this study VO2 increased sharply after surgery reaching the pre-operative VO2 at 1 h with the VO2 peaking at 4 h. It is therefore difficult to understand how anesthesia induces an oxygen debt. This apparent contradiction is best resolved by an analysis of the time course of the mixed venous oxygen saturation (SmvO2) or central venous oxygen saturation (ScvO2) during the peri-operative period. SmvO2 (or ScvO2) is a reflection of the balance between DO2 and VO2; in patients who incur an oxygen debt the SmvO2 should fall. A number of studies have monitored the SmvO2/ScvO2 in the perioperative period [23, 34–39]. These studies reproducibly demonstrate that the SmvO2/ScvO2 remains stable or increases slightly during anesthesia and surgery but falls sharply in the immediate post-anesthesia period. Furthermore, the lowest postoperative SmvO2/ScvO2 was independently predictive of postoperative complications (see Fig. 11.2) [23, 34–38]. These data suggest that the oxygen debt is incurred postoperatively with the withdrawal of anesthesia (and NMB) and with the development of postoperative pain, agitation, shivering and increased sympathetic tone. Furthermore, those patients with limited cardiac reserve and those with inadequate intra-operative hemodynamic optimization are most likely to have the largest postoperative fall in SmvO2/ScvO2 and incur the largest oxygen debt. Indeed, these are the patients that have been demonstrated to be at the greatest risk of death and postoperative morbidity [23, 35].