CHAPTER 75
Percutaneous Peripheral Nerve and Field Stimulation
INDICATIONS
Since the advent of the Gate Control Theory, as proposed by Melzak and Wall, the applications for neurostimulation by stimulation have exploded1. First introduced in the intrathecal space, electrical neuromodulation has progressively been advancing into the periphery, as ablative strategies continue to fall out of favor.
• Peripheral nerve stimulation (PNS) is the direct electrical stimulation of named nerves outside of the neuroaxis.
• Peripheral nerve field stimulation (PNfS) is the stimulation of unnamed small nerves in the vicinity of pain by superficial, subcutaneous lead placement.
• Unlike SCS that modulates second order nociceptors, PNS and PNfS directly inhibit primary nociceptive afferents.
• PNS and PNfS suggest that central sensitization can be subverted by peripheral nociceptive suppression.
• Similar to spinal cord stimulation, therapeutic PNS and PNfS replace the patients pain with a pleasant, therapeutic paresthesia.
Historically, PNS can be performed via an open surgical or percutaneous technique, well described by Stanton-Hicks. The percutaneous techniques for both PNS and PNfS will be reviewed here, while open techniques will not be discussed. Further, although the scope of vagal nerve stimulation has broadened to include the treatment of neuropathic pain, these strategies have not become commonplace and will not be discussed.
• Like spinal cord stimulation, patient selection is crucial to treatment success.
• PNS and PNfS are indicted for chronic neuropathic pain from peripheral origin.
• Peripheral nerve and field stimulation lack strong leveled evidence.
• Percutaneous neuromodulatory stimulation devices are not approved for PNS or PNfS by the FDA and are classified as “off label.”
Not withstanding, the current indications for PNS include:
• Complex regional pain syndrome type II
• Neuropathic pain from mononeuropathy or plexopathies
• Headache (migraine, trigeminal neuralgia, occipital neuralgia, supraorbital neuralgia, cervicogenic headache, hemicrania continua)2
Ideal candidacy for PNS has yet to be determined, as juxtaposed opinions exist. While some advocate a successful nerve block prior to the trial, others contend that a previous successful nerve block is unnecessary. Notwithstanding, a trial prior to implantation is mandatory. With the open technique (not described here), some advocate a direct implant approach in an effort to reduce repetitive procedural morbidity44.
PNfS indications are less clear, as the mechanism is ill-defined.
• Neuropathic and nociceptive pain
• PNfS alone or in concert with epidural leads for treatment of axial back pain related to FBSS3
• Failure or contraindication to conventional and conservative strategies
PNfS candidacy is even less defined and further debated. Failure of conservative and traditional management of neuropathic or mixed nociceptive/neuropathic pain is typically a prerequisite.
Patient selection regarding psychometric testing cannot be understated. Poor treatment outcome have been reported in patients with presurgical somatization, depression, anxiety, and poor coping strategies.
• Comorbid psychiatric illness significantly reduces interventional treatment success rates.
• Approximately 20% to 45% of chronic pain patients have accompanying psychopathology.
• It is paramount to identify candidates suitable for concurrent treatment or exclude patients that require psychiatric treatment primarily.
RELEVANT ANATOMY
Peripheral neuromodulatory success centers on a keen understanding of the anatomy of the peripheral nerve (Figure 75-1).
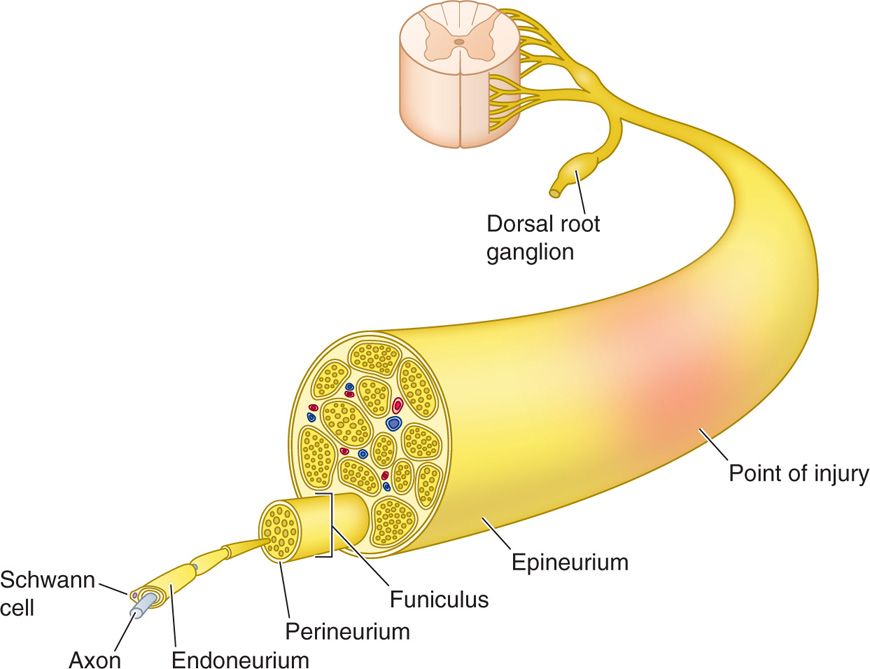
Figure 75-1. Peripheral nerve architecture42.
• Nerves are composed of axons encompassed by Schwann cells, with or without a myelin sheath.
• The cell body of the sensory nerve is located in the dorsal root ganglion and is unipolar.
• Sensory afferent nerve cell bodies extend an axon while dendrites allow synaptic communication with neighboring cells.
• These axons are encased within the endoneurium and bundled into fascicles and surrounded by perineurium.
• These fascicles divide and fuse to form multiple plexi along the nerve trunk, with discrete topographic architecture.
• The vasculature of the peripheral nerve resides in the perineurium.
• The perineural bundles are then finally encased by epineurium.
Peripheral nerves can be categorized based on their conduction velocity and diameter.
Importantly, the aforementioned nerve layers and inconsistent fascicular topographic arrangement provide an anatomic explanation not only for the impendence requirement to overcome, but also the cumulative effect of the cathodal stimulation employed in peripheral neuromodulation.
Relevant anatomy for the common sites of neuromodulation will be discussed in the subsequent sections and include:
• Supraorbital nerve
• Infraorbital nerve
• Greater occipital nerve
• Ulnar nerve
• Median nerve
• Suprascapular nerve
• Intercostal nerve
• Ilioinguinal nerve
• Iliohypogastric nerve
• Genitofemoral nerve
• Lateral femoral cutaneous nerve
• Saphenous nerve
• Sciatic nerve
• Posterior tibial nerve
CONTRAINDICATIONS
• Local infection near the injection site
• Coagulopathy
• Allergy to injectate
• Comorbidities/conditions that prevent fluoroscopic needle guidance
• Inability to obtain consent
• Cognitive impairment with stimulator management
• Coagulopathy8
PREOPERATIVE CONSIDERATIONS
• The use of peripheral nerve stimulation may be more applicable to the patient with multiple comorbidities.
• Use the guidelines and follow the classifications set forth by the American Society of Anesthesiologists for preoperative preparation.
• A clear understanding of surgical site anatomy, utilization of image guidance, and proper surgical technique are vital to ensure both quality treatment and reduced patient morbidity and mortality.
• Proper training is essential to good clinical outcomes.
• Mitigation of infection risk when implanting a foreign body is critical. Risk factors associated with infections of implanted hardware:
![]() Immunosuppressive therapy (including steroids)
Immunosuppressive therapy (including steroids)
![]() Diabetes
Diabetes
![]() Rheumatoid arthritis
Rheumatoid arthritis
![]() Tobacco and/or alcohol use
Tobacco and/or alcohol use
![]() Malnutrition
Malnutrition
![]() Obesity
Obesity
![]() Prolonged hospital stay
Prolonged hospital stay
![]() Multiple surgeries
Multiple surgeries
![]() Perioperative transfusion
Perioperative transfusion
• Reduction is surgical site infections risk factors:
![]() Appropriate selection and timing of preoperative antibiotic therapy
Appropriate selection and timing of preoperative antibiotic therapy
![]() Hair clipping of operative site
Hair clipping of operative site
![]() Proper surgical site skin prep
Proper surgical site skin prep
![]() Adequate preoperative hand scrub
Adequate preoperative hand scrub
![]() Appropriate ventilation in operative suite
Appropriate ventilation in operative suite
• Patient education, informed consent, and patient responsibilities should be completed prior to surgery.
• Percutaneous PNS and PNfS approaches utilize devices that were designed for use in the epidural space.
Documentation for Off-Label Indications
• In most cases, PNS and PNfS are not labeled for use in the neuropathic pain patient. Some devices have achieved labeling in the past, and the issues should be reviewed with the patient prior to moving forward.
Imaging
• The use of fluoroscopy is helpful in orienting the lead position and future issues surrounding migration.
• In large nerve targets, the use of ultrasound has been advocated.
• The choice of imaging should be based on a patient specific characteristics and operator experience.
Position of the Patient
• The position of the patient is dependent on the nerve targeted to promote appropriate surgical site and adequate field margin preparations.
• It may be necessary to reposition the patient during surgery.
Selection of Needles, Medications, and Equipment
• The majority of leads, generators, and tools developed for the spine and has been adopted to use in the periphery10.
• The goal of lead placement is to produce cathodal stimulation of a neural target.
• Although power sources can be either RF coupled or a rechargeable or non-rechargeable IPG (implanted pulse generator), only the RF-coupled transmitter receiver is approved by the FDA for PNS use.
• The needles used for epidural entry for spinal cord stimulation are bent to facilitate percutaneous subcutaneous or peripheral nerve placement, while mitigating needle tip or electrode erosion.
• Unlike spinal cord stimulator lead placement, percutaneous peripheral nerve placement is dependent primarily on needle orientation.
• Stimulation characteristics are similar to SCS stimulation parameters:
![]() Amplitude (strength of the stimulation as determined by volts or amperage)
Amplitude (strength of the stimulation as determined by volts or amperage)
![]() Pulse width (the duration of the stimulation application)
Pulse width (the duration of the stimulation application)
![]() Rate or frequency (the speed at which the stimulation application occurs)
Rate or frequency (the speed at which the stimulation application occurs)
Intraoperative Technical Steps
PNS and PNfS trialing and permanent implantation require a meticulous sterile preparation and wide enough operative fields to visualize the necessary surgical targets. Further, peripheral nerve stimulation is only limited by the ability to visualize the target nerve and IPG implantation location. Peripheral nerve stimulation targets will be discussed separately. Deer described surgical technique for these implants in detail, including common pearls and pitfalls.
PERIPHERAL NERVE STIMULATION
Supraorbital Nerve Stimulation Trial
• The supraorbital nerve, along with the supratrochlear nerve, are terminal branches of the ophthalmic division of the trigeminal nerve.
• The supraorbital nerve exits the supraorbital canal (Figure 75-2).
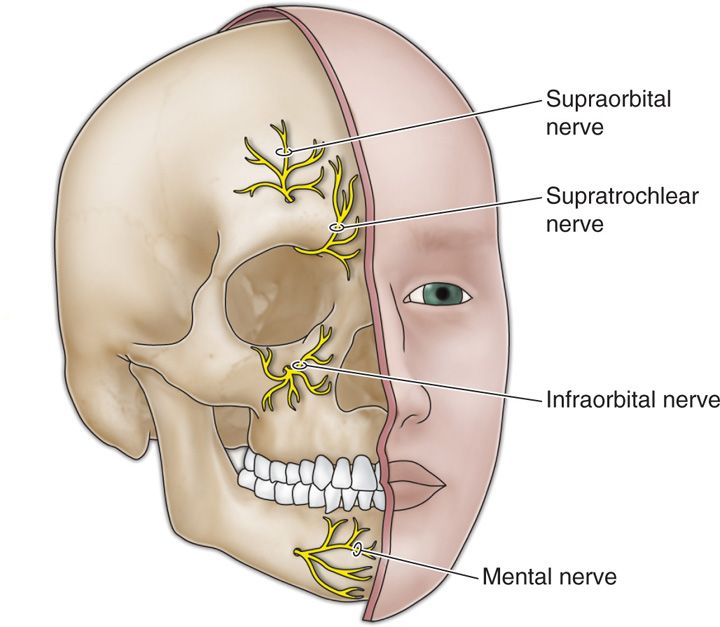
Figure 75-2. Terminal branches of the trigeminal nerve12.
• Slavin et al described the most commonly employed technique for supraorbital stimulation9.
• The patient is positioned supine, standard prep and drape (alcohol should be avoided in the face to avoid corneal irritation).
• Monitored Anesthesia Care is suggested.
• Fluoroscopy is used in the anterior-posterior view to approximate the target.
• A skin wheel using 1% lidocaine is raised approximately 3 to 4 cm lateral to the lateral corner of the eye and along the needle tract.
• A stab incision is then made, where a standard 14-gauge Tuohy needle (bent to allow and follow the contour of the face), is directed toward the midline approximately 1 cm above the supraorbital ridge until it is approximately 1 cm from the midline (Figure 75-3).
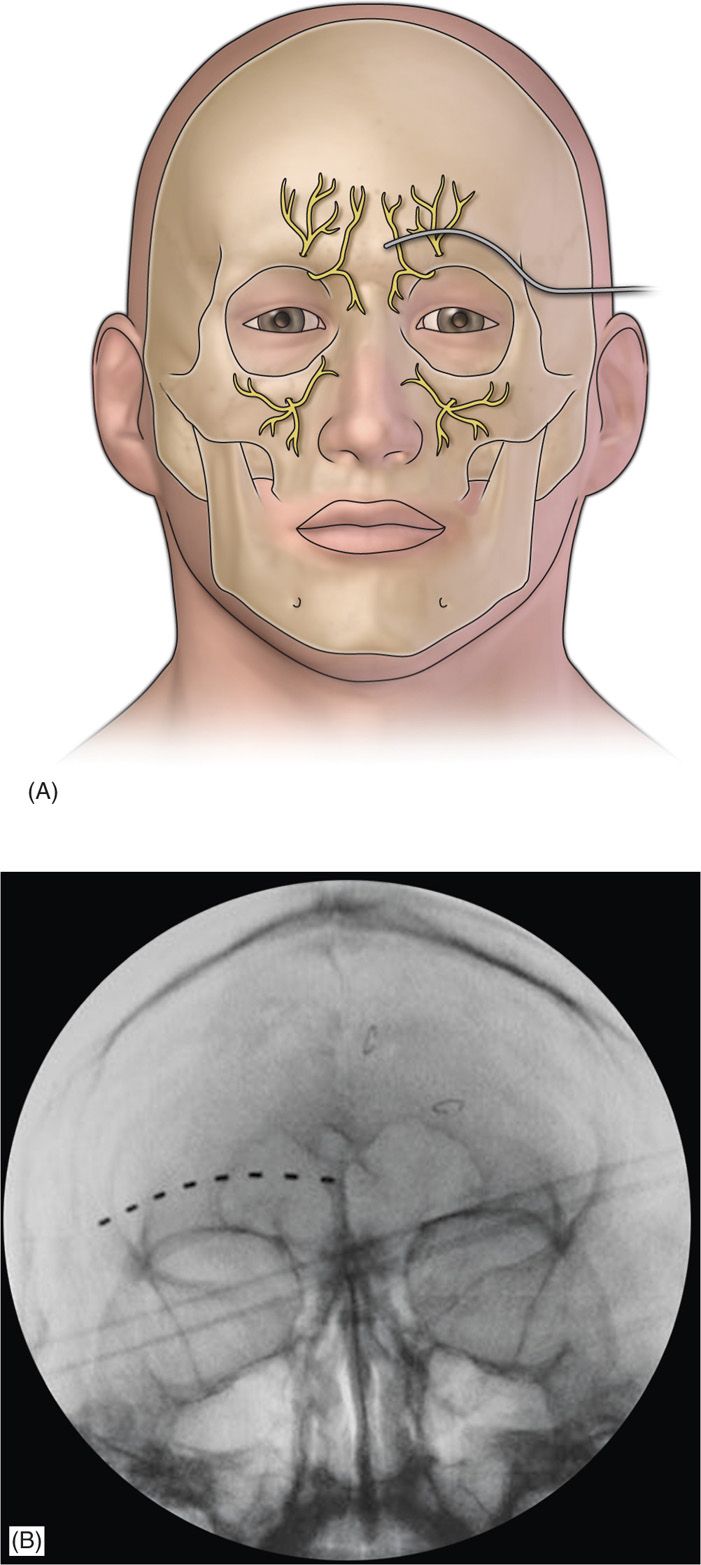
Figure 75-3. Diagram of supraorbital lead placement13 (A) and AP radiograph of electrode under fluoroscopy (B)14. (Reproduced with permission from Johnson RD, Green A, Aziz TZ. Implantation of an intercostal nerve stimulator for chronic abdominal pain. The Royal College of Surgeons of England. 2010. 92, 1-3.)
• Care must be taken not to direct the needle too superficially, as this may promote lead tip erosion.
• The stylet is removed, the percutaneous electrode is placed, and the needle is withdrawn to allow for intraoperative stimulation testing.
• Judicious use of local anesthetic at the puncture site will allow for intraoperative testing, while needle track topicalization to the midline target will likely preclude intraoperative testing.
• The externalized lead is then secured with the supplied plastic anchor of the surgeons choosing and nonabsorbable sutures.
• A sterile dressing is applied and the patient is taken to the recovery area.
Infraorbital Nerve Stimulation Trial
• The infraorbital nerve is one of the terminal branches of the maxillary division of the trigeminal nerve and exits via the infraorbital canal (Figure 75-2).
• The patient is prepared, positioned, and anesthetized as for supraorbital stimulation.
• The target site is the infraorbital foramen on fluoroscopy.
• The lead is placed approximately 1 cm below the orbit and just lateral to the ipsilateral nose, as described by Slavin et al.
• The entry point is lateral and inferior to the eye over the zygoma.
• After judicious local anesthetic use at the entry point and topicalization of the needle tract, a bent introducer needle to accommodate the contour of the face is inserted and directed to the target zone under fluoroscopy.
• Once the needle is in the correct position, the percutaneous cylindrical lead is introduced with care not to direct the distal tip of the needle too superficial to avoid lead tip erosion.
• The introducer needle is withdrawn (Figure 75-4).
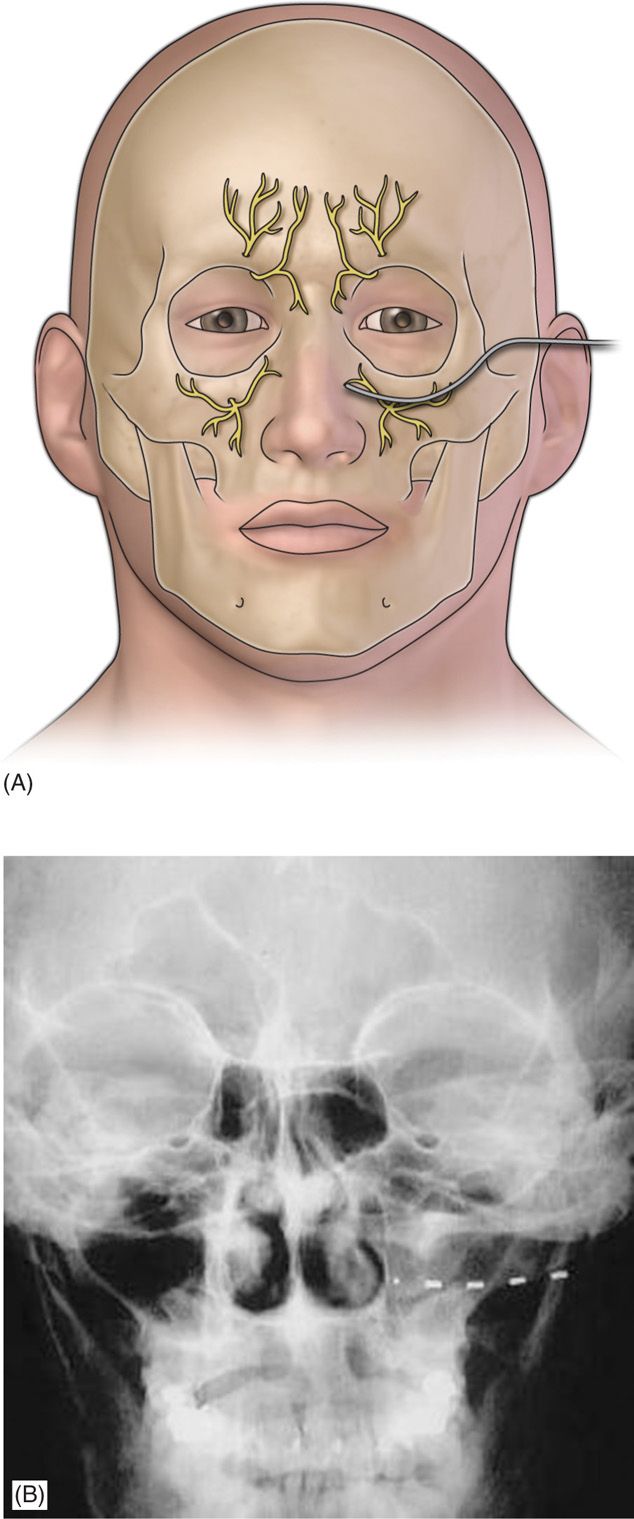
Figure 75-4. Diagram of Infraorbital lead placement13. (Reproduced with permission from Johnson RD, Green A, Aziz TZ. Implantation of an intercostal nerve stimulator for chronic abdominal pain. The Royal College of Surgeons of England. 2010. 92, 1-3.)
• The lead is sutured as previously described and a sterile dressing is applied.
Supra- and Infraorbital Nerve Permanent Implant
• For the permanent implantation of both the infra and supra orbital leads and IPG, general anesthesia is recommended (with laryngeal mask airway if feasible).
• The patient is positioned supine with a slight contralateral head turn to provide access to the retroauricular location.
• A meticulous sterile prep and drape is required to accommodate tunneling and IPG site location. Commonly, the infraclavicular sight is chosen.
• The permanent percutaneous lead is inserted as described for the trial.
• Additional incisions are made in the ipsilateral retroauricular location after appropriate topicalization.
• Two techniques have been described for tunneling the lead’s IPG connection portion:
![]() One is simply using the introducer needle with stylet in place (bent to accommodate the contour of the head and tunneling from the retroauricular incision to the anterior incision). The stylet is then removed, the lead introduced, and the needle withdrawn, leaving the tunneled lead.
One is simply using the introducer needle with stylet in place (bent to accommodate the contour of the head and tunneling from the retroauricular incision to the anterior incision). The stylet is then removed, the lead introduced, and the needle withdrawn, leaving the tunneled lead.
![]() Another technique is the “needle-over-the-stylet” technique, as described by Slavin et al. The stylet of the needle is tunneled from the anterior to the posterior incision and then the needle is passed over the stylet toward the anterior incision. The stylet is then removed and the lead is inserted in the needle tunneling toward the retroauricular incision. The needle is then removed, leaving the lead in place.
Another technique is the “needle-over-the-stylet” technique, as described by Slavin et al. The stylet of the needle is tunneled from the anterior to the posterior incision and then the needle is passed over the stylet toward the anterior incision. The stylet is then removed and the lead is inserted in the needle tunneling toward the retroauricular incision. The needle is then removed, leaving the lead in place.
• From the retroauricular location, the lead is secured with the supplied plastic anchor using nonabsorbable suture.
• A stress loop is recommended (~2-3 cm in diameter) and the lead is attached to the extension cable.
• Place the extension cable connection in close approximation to the retroauricular incision to allow for easy access if reoperation is required.
• The IPG site location is largely the surgeon’s preference.
• Flexion and extension pathway changes in the infraclavicular and abdominal sites were associated with less length changes as compared to the periscapular and gluteal sites, respectively.
• The lead extensions placed that span mobile areas, including joints, will have a higher propensity to migrate.
• Avoid IPG placement around osteal structures to reduce pain overlying the IPG device.
• It is crucial to avoid excessive blunt dissection, provide meticulous hemostasis, and anchor the perimuscular fascia to avoid IPG dislodgement.
• Copious nonpressurized irrigation is performed at all incision sites and layered closure is performed with absorbable suture.
• Avoid placing sutures overlying the IPG, as this may impair wound healing and increase the chance of wound dehiscence.
• Sterile dressing is applied and the patient is recovered in the postoperative area.
Greater Occipital Nerve Stimulation Trial
• The greater occipital nerve is also known as the second occipital nerve and is the medial branch of dorsal primary rami of C2.
• Since the characterization of the trigeminocervical complex, the greater occipital nerve has become a popular target for the treatment of headache.
• Anatomic dissection studies have been performed to characterize the location from osteal landmarks, including the mastoid process and the occipital protuberance.
• It is generally found 1.4 to 1.6 cm lateral from the external occipital protuberance and 2.91 to 3.7 cm inferior.
• The nerve is consistently medial to the occipital artery (Figure 75-5).
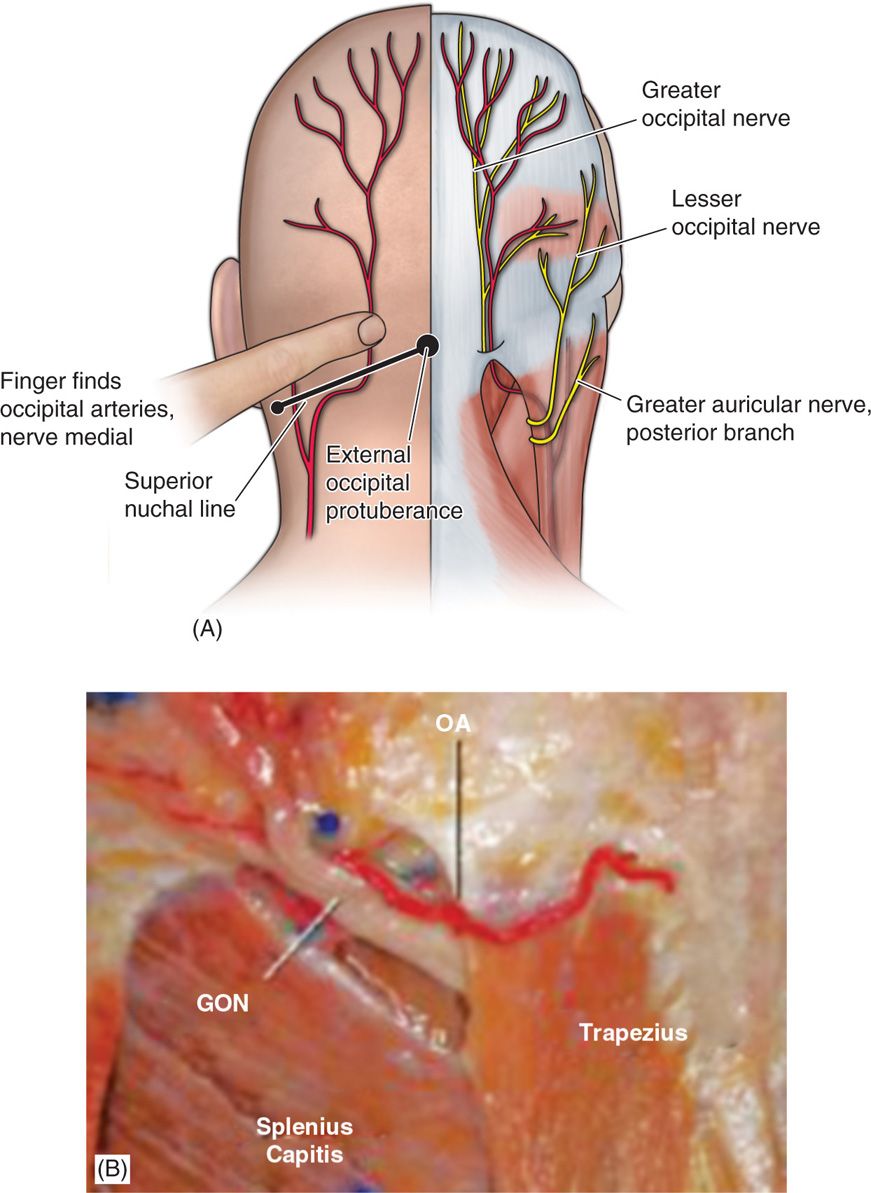
Figure 75-5. Greater occipital nerve diagram12 and anatomic dissection.
• There are multiple techniques described to stimulate the greater occipital nerve, with major differences centering on target location:
![]() C1-C2 versus nuchal ridge/retromastoid
C1-C2 versus nuchal ridge/retromastoid
![]() Lead trajectory orientation (medial to lateral or lateral to medial)
Lead trajectory orientation (medial to lateral or lateral to medial)
![]() Type of lead chosen (percutaneous cylindrical vs paddle) (Figure 75-6)
Type of lead chosen (percutaneous cylindrical vs paddle) (Figure 75-6)
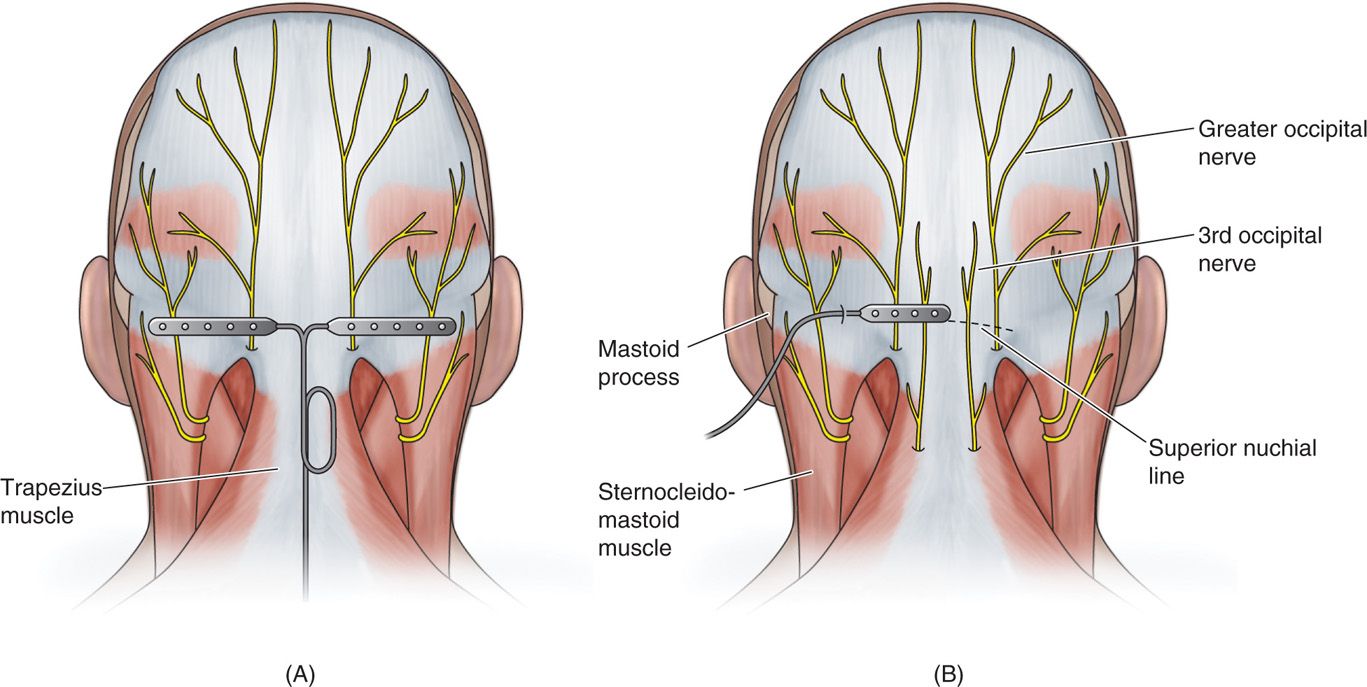
Figure 75-6. Diagrams of percutaneous and paddle ONS lead placements19, 20, 21.
• Muscle spasm may be subverted by placement of the electrodes at the nuchal line, as opposed to the C1-C2 level.
• Superficial lead placement may increase the chance of erosion and burning sensations, while too deep lead placement may cause aberrant muscular stimulation.
• The trial procedure is carried out with the patient in the prone position.
• Monitored anesthesia care or sedation is suggested for the trial.
• The surgical site is prepared by hair clipping (not shaving), and meticulous sterile prep and drape in the normal fashion, leaving the entry site exposed.
• Image guidance is a prerequisite; fluoroscopy is commonly employed.
• After the target location is chosen (AA joint vs superior nuchal line), the incision site is identified.
• Do not to anesthetize the greater occipital nerve, and use judicious local anesthetic at the incision site using 1% lidocaine.
The medial, nuchal line approach (Figure 75-7):
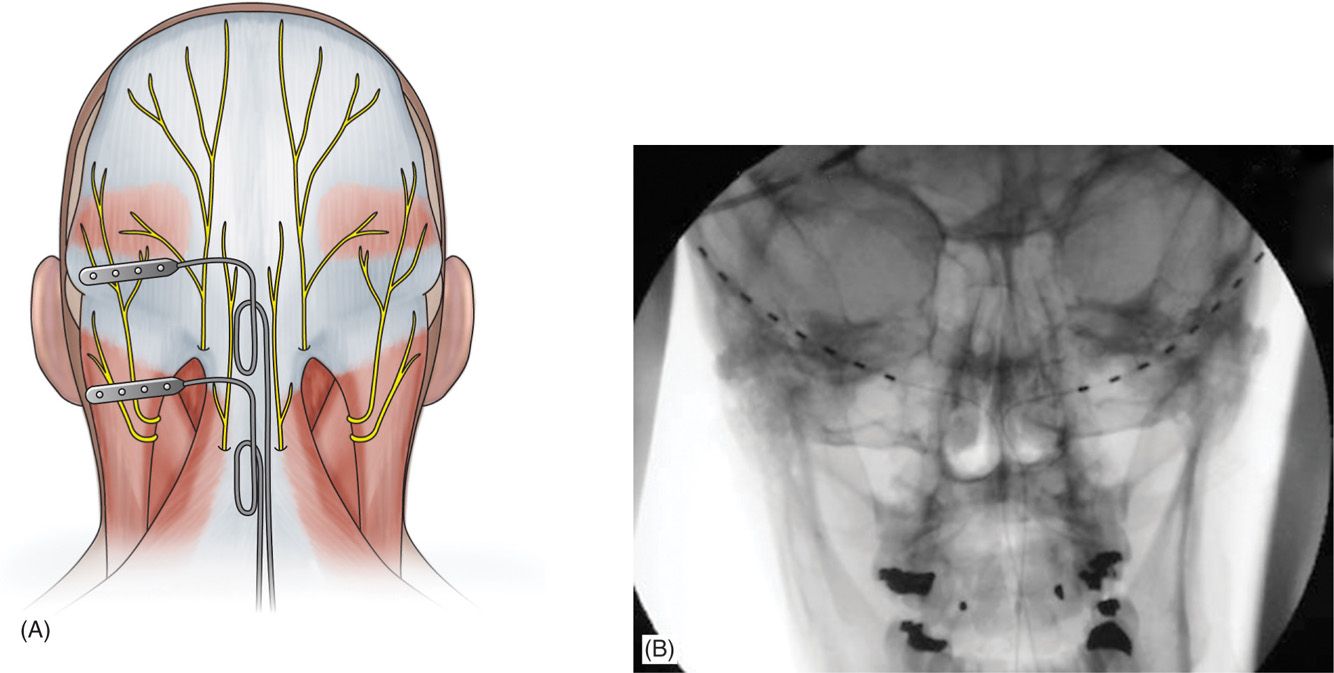
Figure 75-7. Percutaneous lead site differences19. (Reproduced with permission from Hayek SM, Jasper JF, Deer TR, Narouze SN. Occipital Neurostimulation-Induced Muscle Spasms: Implications for Lead Placement. Pain Physician 2009. 12:867-876.)
• An incision is made in the midline just caudal to the occipital protuberance.
• The needle is bent to accommodate the contour of the head.
• Under fluoroscopic guidance, lead is placed along the nuchal ridge ipsilateral to the target greater occipital nerve.
• Once appropriate lead position is achieved, the needle is withdrawn slightly to allow for intraoperative stimulation testing if desired.
• The needle is removed and the lead is secured using the plastic anchor provided and nonabsorbable suture.
• A sterile dressing is applied and the patient is further recovered and programmed in the recovery room.
Greater Occipital Nerve Permanent Implant
• Preparation and anesthesia, and the lead placement procedure are largely the same for the occipital nerve trial and implant.
• The surgical prep site is extended to a larger area to accommodate the IPG location.
• Like SCS, strain loops are created at the incision site by careful lateral dissection.
• Plastic anchors and nonabsorbable sutures are used to suture the lead to the dorsal fascia.
• A third incision is made and carefully dissected to accommodate the IPG.
• If extensions are needed and tunneling is required over a great distance, additional incisions with sequential tunneling may be required.
• Irrigation is performed at all incision sites and layered closure is recommended.
• Do not create a suture line overlying the implanted device.
• Sterile dressings are applied and further programming is performed in the recovery area.
UPPER AND LOWER EXTREMITY PERIPHERAL NERVE STIMULATION
The feasibility of distal upper and lower extremity peripheral nerve stimulation was investigated by a series of articles by Huntoon et al, describing techniques for ulnar, median, radial, peroneal, and the posterior tibial nerves. Careful identification of the target nerves and familiarity with ultrasound-guided techniques cannot be understated. Formal accredited fellowship training is recommended. Some examples of peripheral nerve stimulation techniques are described below.
Ulnar Nerve Stimulation Trial
• The ulnar nerve is the most caudal portion of the brachial plexus, arising from the medial cord with nerve roots originating at C8-T1.
• It descends medially to the brachial artery in the proximal arm, anterior to the medial triceps of the triceps, and it resides in the grove of the medial epicondyle at the elbow.
• Patient is positioned supine and patient preparation performed in the usual manner including sterile prep and preoperative antibiotics.
• As described by Huntoon et al, the easily identified ulnar nerve located at the medial epicondyle is traced in the axial sonographic view to approximately 9 to 13 cm proximal.
• Once the nerve was located, a skin wheel is raised with lidocaine 1%, and a skin nick is created.
• Under a live axial view of the ulnar nerve, the needle is then introduced vial the long axis of the probe, placing the lead deep, adjacent and perpendicular to the ulnar nerve.
• The needle is retracted and stimulation testing commenced.
• Anchoring of the lead to the skin was performed using the plastic anchors and nonabsorbable suture (Figure 75-8).
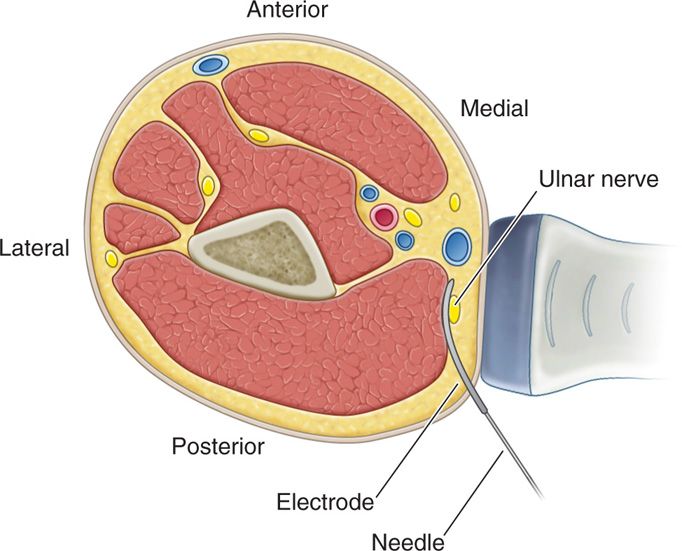
Figure 75-8. Diagram depicting US guided peripheral nerve stimulation6.
Median Nerve Stimulation Trial
• The median nerve arises from the C5, C6, C7, C8, and T1 roots, and is more distally from the lateral and medial cords.
• It descends anterolateral to the axillary and brachial artery, lies medial to it and the biceps muscle tendon in the cubital fossa.
• Moving distally in the forearm, the median nerve descends between the heads of the pronator teres muscle, and in the wrist it resides between the tendons of the flexor carpi radialis and the flexor digitorum superficialis (Figure 75-9).
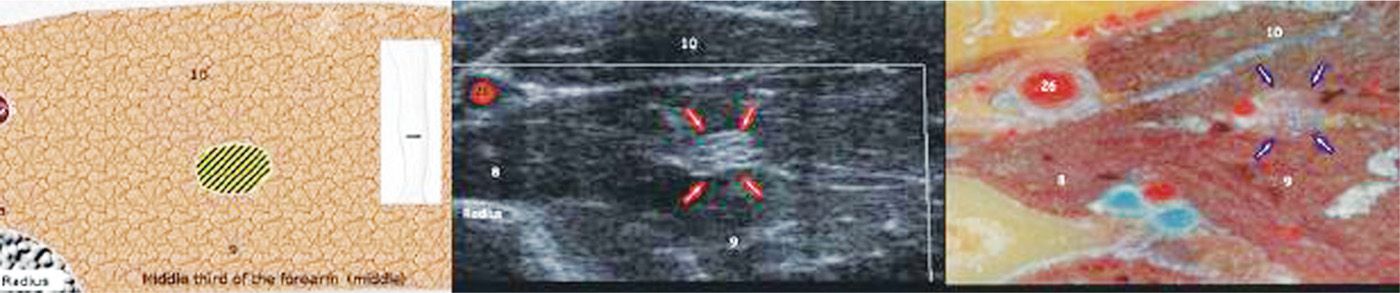
Figure 75-9. Median nerve axial view proximal forearm27. (Reproduced with permission from Bargallo X, Carrera A, Sala-Blanch X, Santamaria G, Morro R, LLusa M, Gilabert R. Ultrasound-Anatomic Correlation of the peripheral nerves of the upper limb. Surgical Radiol Anat 2010. 32:305-314.)
• The patient is positioned supine and preparation is performed in the usual manner.
• The ultrasound probe is placed in the transverse position and scanned distally until approximately 4 to 6 cm distal to the antecubital fossa between the pronator teres heads.
• After careful topicalization with 1% lidocaine at the incision site only, a skin incision is made and under ultrasound guidance.
• A 14-gauge needle is introduced in the longitudinal plane with the target nerve and maintained in the axial plane with care to place the lead adjacent to the nerve.
• Once the lead is in the optimal position, the needle is retracted and stimulation testing is performed.
• After stimulation is achieved, anchoring of the lead to the skin is performed using the plastic anchors and nonabsorbable suture.
• A sterile dressing is applied. The patient is then transported to the recovery room for further programming.
Radial Nerve Stimulation Trial
• The radial nerve has origins from the posterior cord from roots C5-C8. The nerve travels obliquely to the humerus at in the proximal arm, along with the deep brachial artery.
• The nerve is reliably located lateral to the humerus at approximately 10 to 14 cm proximal to the lateral epicondyle deep to the lateral head of the triceps (Figure 75-10).
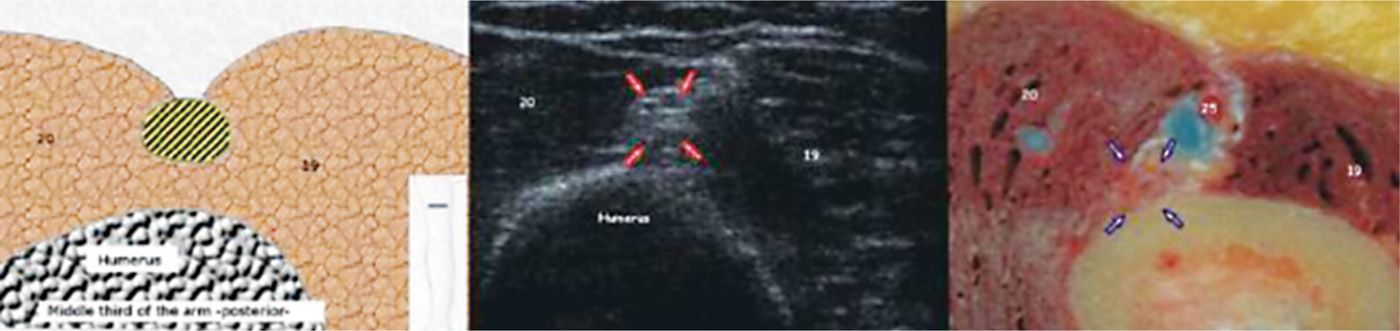
Figure 75-10. Radial nerve in the proximal arm27. (Reproduced with permission from Bargallo X, Carrera A, Sala-Blanch X, Santamaria G, Morro R, Llusa M, Gilabert R. Ultrasound-Anatomic Correlation of the peripheral nerves of the upper limb. Surgical Radiol Anat. 2010;32:305-314.)
• The patient is positioned supine and preparation is performed in the usual manner.
• The ultrasound probe is placed in the transverse position and scanned distally until approximately 10 to 14 cm proximal to the lateral epicondyle.
• After careful topicalization with 1% lidocaine at the incision site only, a skin incision is made and under ultrasound guidance.
• A 14-gauge needle is introduced in the longitudinal plane with the target nerve maintained in the axial plane with care to place the lead adjacent to the nerve.
• Once the lead is in the optimal position, the needle is retracted and stimulation testing is performed.
• Once therapeutic stimulation is achieved, anchoring of the lead to the skin is performed using the plastic anchors and nonabsorbable suture to the skin.
• A sterile dressing is applied, and the patient is then transported to the recovery room for further programming.
Median, Ulnar, and Radial Permanent Implant
• The patient preparation, anesthesia, and placement of the lead are the same as the trial procedure.
• The surgical prep site is extended to a larger area to accommodate the IPG location.
• For the permanent percutaneous placement, it is recommended to place the device in the upper chest (infraclavicular site) or abdomen for the upper extremity.
Lead extensions and serial incisions are required to connect and tunnel the leads to the IPG. As described previously, meticulous surgical technique is paramount, including appropriate hemostasis and dissection to the fascia for lead anchoring. Sterile dressings are applied and programming is performed in the recovery room.
Peroneal Nerve Stimulation Trial
• The sciatic nerve is formed from the L4 to S3 nerve roots and can be subdivided into medial and lateral compartments.
• The medial portion of the sciatic nerve is functionally the tibial nerve, formed by the ventral branches of the L4-L5 and S1-S3, while the posterior branches of the ventral rami make up the peroneal nerve.
• The sciatic nerve descends in the rostral portion of the popliteal fossa, splitting into the tibial nerve medially and the common peroneal nerve laterally.
• The popliteal fossa’s borders are the semimembranosus and semitendinosus medially, the biceps femoris laterally, and the gastrocnemius muscle caudally.
• The popliteal artery is medial to the neural targets (Figure 75-11).
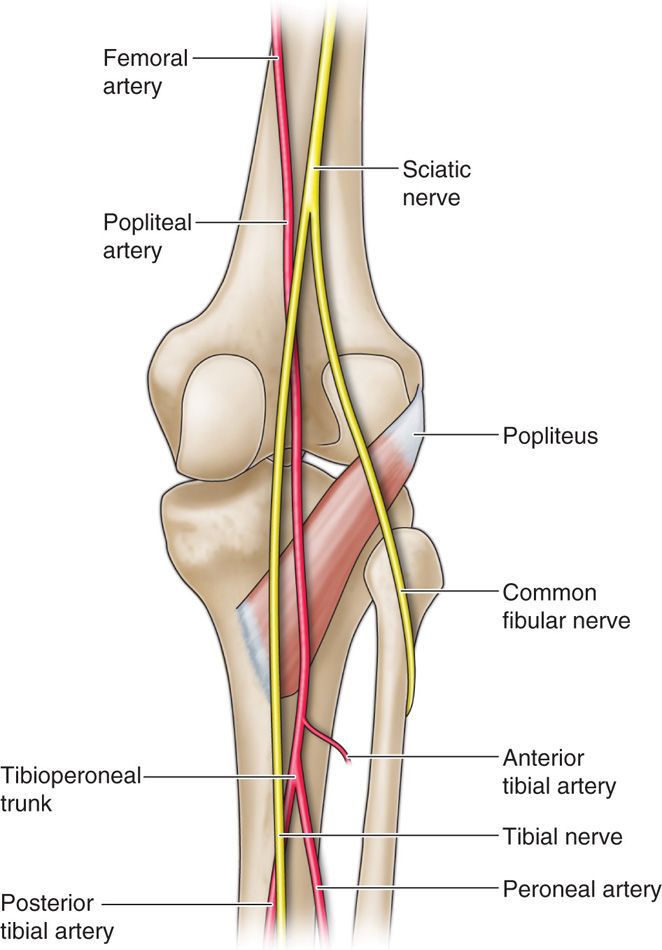
Figure 75-11. Popliteal fossa41; saphenous and sural cutaneous branch stimulation.
• The patient is positioned to access the popliteal fossa of the afflicted leg. After patient preparation, monitoring, and meticulous sterile prep and drape, as previously described.
• Axial ultrasound scanning is performed from the popliteal crease cephalad. The tibial and peroneal nerves coalesce to form the sciatic nerve just cephalad to the aforementioned popliteal fossa.
• Identification of the popliteal artery is essential to avoid vascular entry. Once the desired nerve location is visualized, judicious topicalization of the skin entry site with 1% lidocaine is performed.
• The introducer needle is then placed deep to the bifurcation of the sciatic nerve in a posterolateral to anteromedial direction.
• Be careful to avoid muscular entry is essential. The lead is introduced and the needle is retracted to allow for testing.
• Once therapeutic testing is achieved, the needle is withdrawn, the lead sutured to the fascia of the biceps femoris muscle using the plastic anchor and nonabsorbable suture.
• Sterile dressing applied, and the patient transported to the recovery room for further stimulation testing.
Saphenous Nerve Simulation Trial
• The saphenous nerve is a purely sensory nerve that is a distal cutaneous branch of the femoral nerve and therefore has contributions from the L2-L4 nerve roots.
• It descends along the medial aspect of the thigh and posterior to the sartorius muscle.
• In the distal thigh, the nerve lies between the tendons of the sartorius and gracilis muscles (or vastus medialis muscle more distally), where it can be reliably located just proximal to the medial aspect of the knee and approximates the geniculate artery (Figure 75-12).
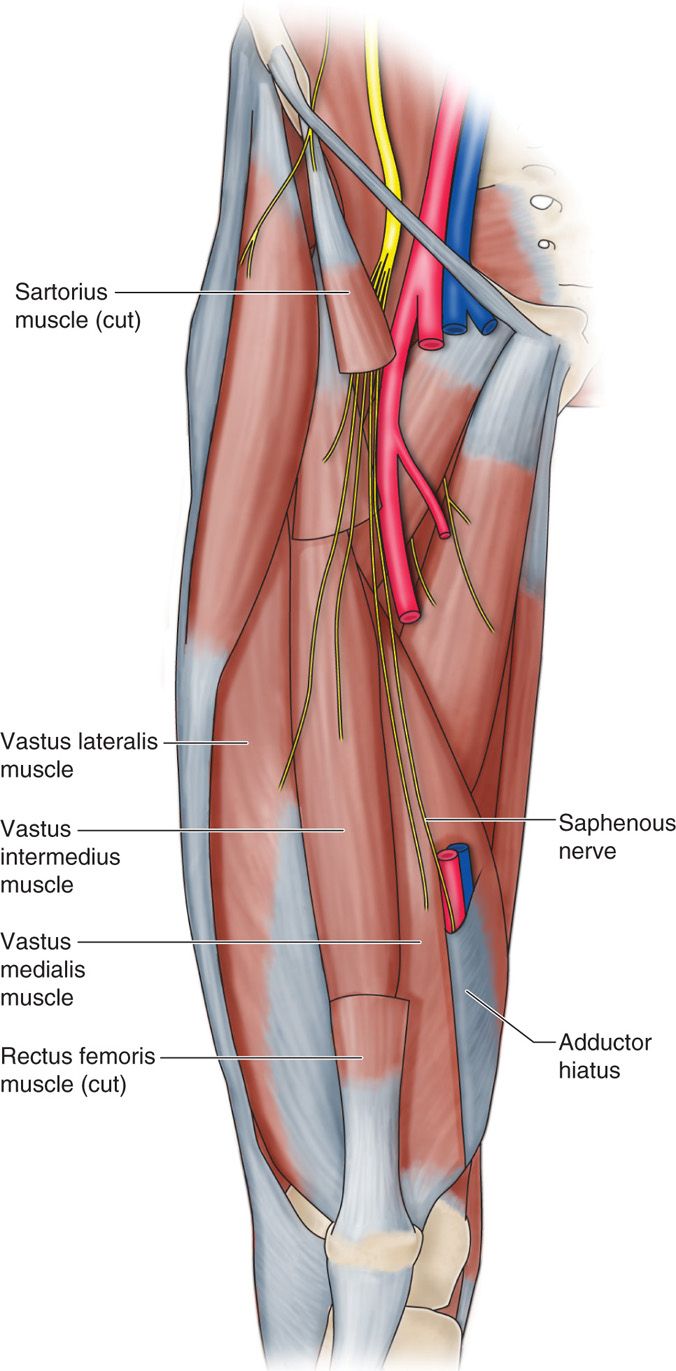
Figure 75-12. Depiction of saphenous nerve28.
• The patient is positioned supine with slight ipsilateral extremity hip external rotation.
• Appropriate patient preparation is carried out in usual fashion.
• Axial scanning of the afflicted extremity performed for an anatomic survey. The saphenous nerve is predominately hyperechoic and Doppler survey to identify the geniculate artery may help identifying the target.
• After needle entry topicalization with 1% lidocaine, a small skin incision is made.
• A 14G introducer needle is introduced and directed to the facial plane between the sartorius muscle and vastus medialis using an in-plane approach, avoiding muscle penetration.
• Once the needle approximates the nerve, the stimulation lead is placed and the needle retracted to allow for stimulation testing.
• Once therapeutic stimulation is achieved, the needle is removed and the lead is sutured to the vastus medialis fascia using a plastic anchor and nonabsorbable suture.
• A sterile dressing is applied and the patient is transported to the recovery area for further programming.
Lateral Femoral Cutaneous Nerve (LFCN) Stimulation Trial
• The lateral femoral cutaneous nerve is a branch of the posterior divisions of the L2-L3 nerve roots and is also an exclusively sensory nerve.
• It travels lateral to the border of the psoas muscle and courses toward the anterior inferior iliac spine (ASIS).
• It then passes under the inguinal ligament, lying between the fascia lata (deep) and iliaca (superficial), providing sensory information from the lateral thigh.
• LFCN neuropathy is called meralgia paresthetica.
• The patient is positioned supine with slight ipsilateral extremity in neutral position.
• After appropriate patient preparation, monitoring, and field prep and drape, axial scanning of the afflicted extremity performed for an anatomic survey from the ASIS along the inguinal ligament.
• After appropriate needle entry site topicalization, a stab incision is made.
• A 14-gauge needle is introduced superficially along the longitudinal axis of the probe in close proximity to the lateral femoral cutaneous nerve just caudal to the inguinal ligament.
• Once needle placement is optimized, the lead is introduced, placing the lead perpendicular to the course of the nerve.
• The needle is retracted and therapeutic stimulation is achieved.
• The lead is anchored to the fascia lata with the supplied plastic anchor and nonabsorbable suture.
Intercostal Nerve Stimulation Trial
• Intercostal nerves originate as the anterior rami of the paired exiting nerve roots and travel under the adjacent rib with close approximation to the intercostal vein and artery.
• Care must be taken not to violate the pleura.
• After aseptic preparation and monitoring as described previously, the patient is positioned either prone or in the lateral decubitus position.
• Under fluoroscopic guidance, and 1% lidocaine skin wheel, a stab incision is made to accommodate the introducer needle.
• The needle should be bent to follow the curve of the rib.
• Once the needle is verified to be in the correct location, the lead is inserted and the needle retracted for stimulation testing.
• Once therapeutic stimulation is achieved, the lead is anchored with the supplied plastic anchor and nonabsorbable suture (Figure 75-13).
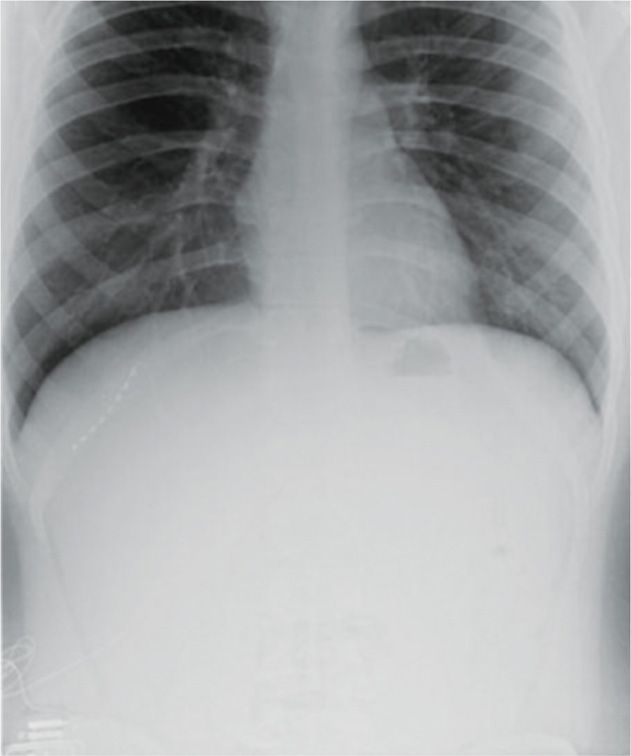
Figure 75-13. Radiograph of T11 intercostal nerve percutaneous PNS40. (Reproduced with permission from Johnson RD, Green A, Aziz TZ. Implantation of an intercostal nerve stimulator for chronic abdominal pain. The Royal College of Surgeons of England. 2010. 92, 1-3. Copyright the Royal College of Surgeons of England.)
Iliohypogastric, Ilioinguinal, Genitofemoral Nerves
• The iliohypogastric and ilioinguinal nerves both arise from the L1 nerve root and emerge lateral to the psoas muscle.
• The nerves course in the anatomic plane of the internal oblique and transversus abdominal muscles.
• The genitofemoral nerve arises from the L1 and L2 nerve roots and emerges on the anterior surface of the psoas muscle.
• It’s genital branch travels through the inguinal canal and in males supplies sensory information from the scrotal skin.
• In contrast, the ilioinguinal nerve supplies the groin.
• These nerves are amenable to peripheral stimulation, and care must be taken to ensure appropriate needle placement without violating the peritoneal cavity; image guidance via ultrasound is recommended.
• As these nerves are difficult to locate, the line between PNS and PNfS begins to blur (Figure 75-14).
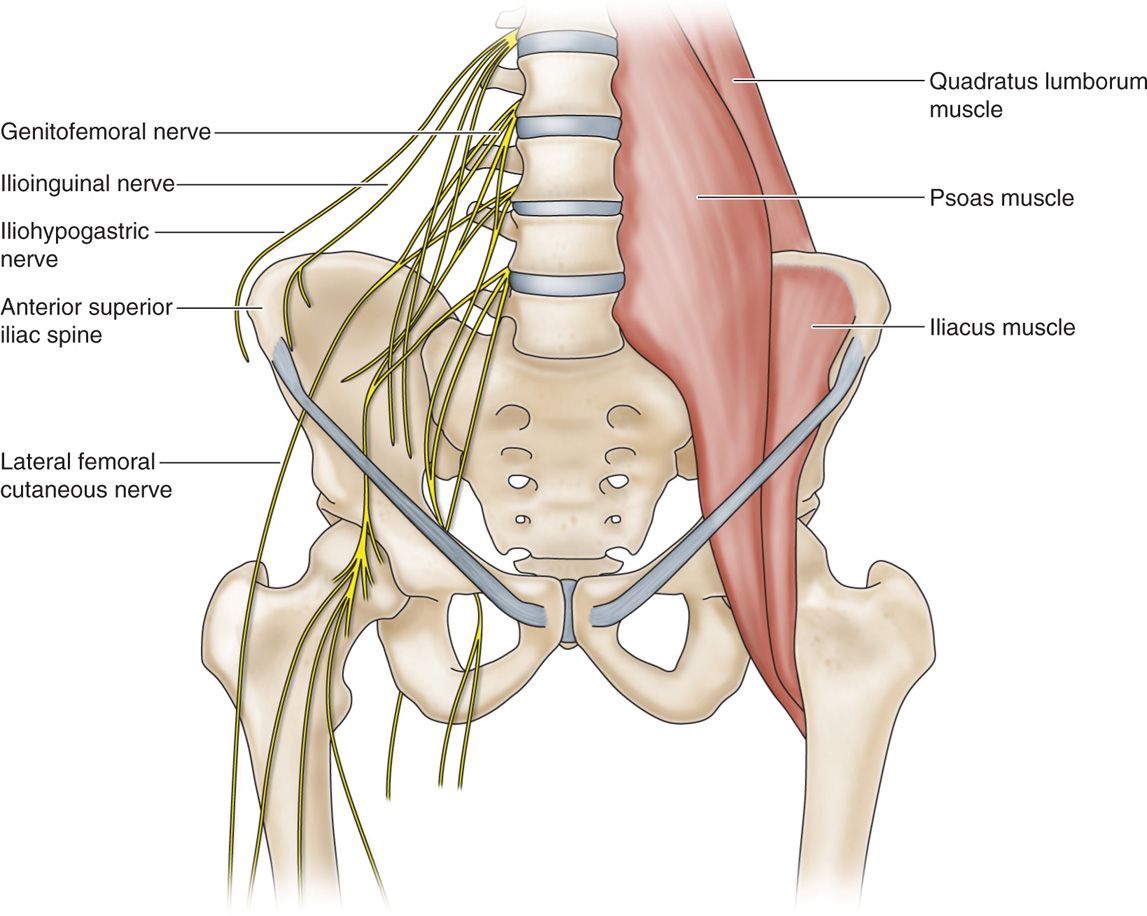
Figure 75-14. (A) Diagram of ilioinguinal hypogastric, and genitofemoral courses12; (B) radiograph ilioinguinal nerve stimulation.
PERIPHERAL NERVE FIELD STIMULATION (PNFS)
As described previously, there is poor prospective data justifying PNfS. Nevertheless, the available evidence does suggest some efficacy in treating chronic neuropathic pain syndromes. PNfS has also been used in conjunction with SCS to treat both back and leg pain, with inter and intra lead programming.
Common areas where PNfS has been employed include:
• Axial thoracic and lumbar back pain
• Failed back surgery syndrome (FBSS)
• Greater trochanteric pain after total hip arthroplasty
• Postherniorrhaophy pain
• Chronic abdominal pain
• Knee pain
• Post-thoracotomy pain
Peripheral Field Stimulation Trial
• The patient preparation and anesthesia are the same for the aforementioned named peripheral nerve neuromodulatory targets.
• Surgical site preparation is dependent on the area of the painful area, and therefore the patient position needs to accommodate any easy operative field access.
• The leads can be placed with different strategies:
![]() Placed to “bracket” the area of neuropathic pain, with the area of coverage is approximately 180 × 90 mm.
Placed to “bracket” the area of neuropathic pain, with the area of coverage is approximately 180 × 90 mm.
![]() Leads placed centrally in the painful area.
Leads placed centrally in the painful area.
• The placement technique depends on infiltration of local anesthetic either at skin entry site only or the entry site and the desired needle tract.
![]() To allow for intraoperative testing
To allow for intraoperative testing
![]() To place the lead without intraoperative testing
To place the lead without intraoperative testing
• A stab incision is created and the 14-gauge introducer needle is inserted near the target area subcutaneously under image guidance.
• After needle position finalized, the percutaneous lead is introduced, the needle is withdrawn, and stimulation testing commences.
• If unpleasant and burning sensations are reported, the lead is likely too deep and needs to be redirected more superficially.
• The lead is then secured to the skin using a plastic anchor and nonabsorbable suture and a sterile dressing is applied.
• The externalized lead is then connected to the externalized battery and the patient is transported to the recovery room for more complex programming (Figures 75-15 to 75-17).
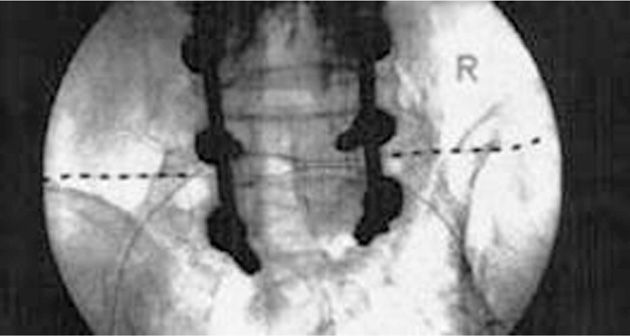
Figure 75-15 PNfS for FBSS34. (Reproduced with permission from Paicius, RM, Bernstein CA, Lempert-Cohen C. Peripheral Nerve Field Stimulation for the Treatment of Chronic Low Back Pain: Preliminary Results of Long-Term Follow-up: A Case series. Neuromodulation 2007. Vol 10, No 3, 279-89.)
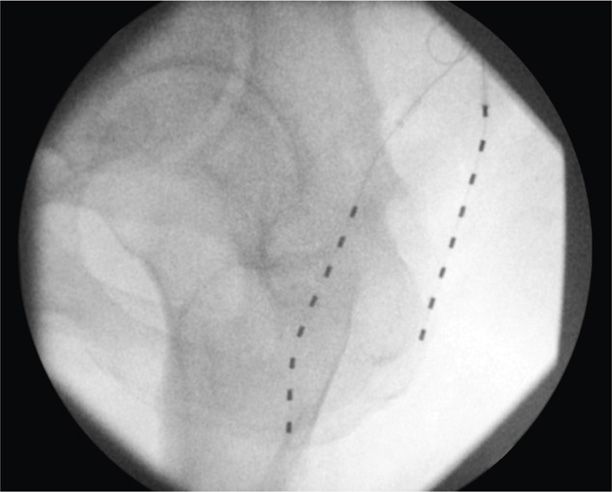
Figure 75-16. PNfS for thigh pain following greater trochanteric bursectomy32. (Reproduced with permission from Yakovlev AE, Resch BE, Karasev SA. Treatment of Intractable Hip Pain after THA and GTB Using Peripheral Nerve Field Stimulation: A Case Series. Wisconsin Medical Journal. 2010, Vol 109 No 3, 149-152.)
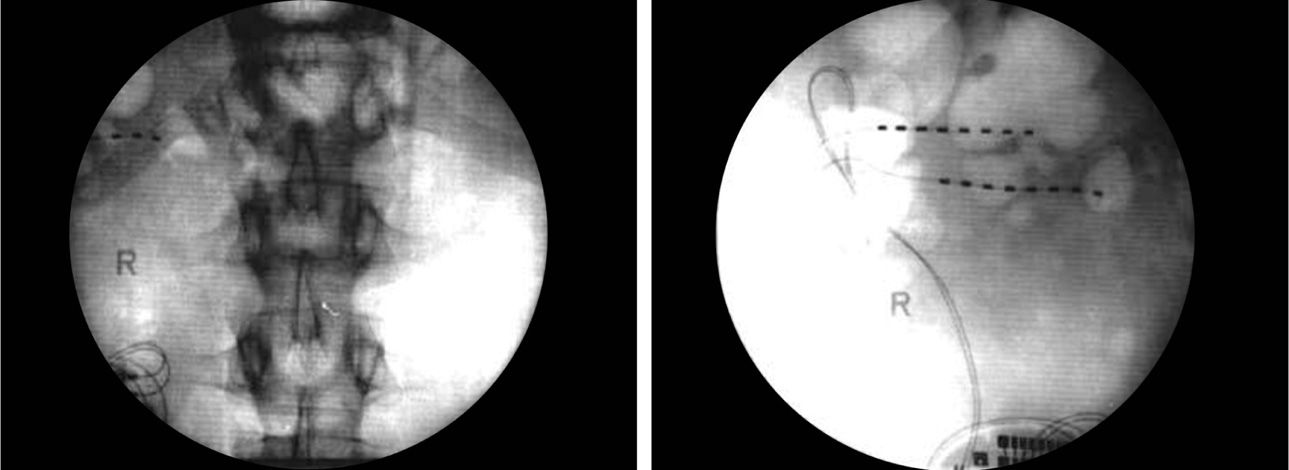
Figure 75-17. PNfS for chronic pancreatitis33. (Reproduced with permission from Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral Nerve Field Stimulation in Chronic Abdominal Pain. Pain Physician. 2006, 261-66.)
Peripheral Field Stimulation Permanent Implant
The peripheral nerve implant following a successful trial proceeds in the same manner as the trial with the additional prep and draping to include the battery site. The strategies and techniques that have been described previously can be translated to PNfS permanent placement.
POSTPROCEDURE CONSIDERATIONS
The decision to provide postoperative prophylactic antibiotics continues to be a controversial topic. Many practitioners continue to do so despite only anecdotal evidence. Monitor for postoperative infection critically.
The signs of infectious complications are:
• Surgical site tenderness
• Accompanied erythema
• Fever of 101.4 or over
• Induration
• Purulent discharge
• Wound dehiscence
Infection if confined locally, antibiotics may be utilized to salvage the device. If systemic signs are suspected, all hardware should be removed, an infectious disease consult sought, and the infection treated and cleared for 6 to 12 weeks before reimplantation is attempted.
• Lead placement too superficial causes a burning sensation, while too deep create muscle recruitment.
• Therapeutic programming should be performed to optimize the devices therapeutic potential and should be adjusted as needed.
• Successful trial stimulation is defined as at least 50% pain reduction and/or 50% improvement in function.
• Trial periods last commonly for 5 to 7 days.
• PNS trials may be better tolerated for longer periods, as there is very low procedural morbidity or mortality innate to the superficial nature of the device placement.
• Once a trial is terminated and deemed successful, 3 to 4 weeks is usually allowed before permanent device placement.
MONITORING OF POTENTIAL COMPLICATIONS
Potential complications include:
• Infection
• Lead migration
• Lead tip erosion
• Overstimulation
• Hardware malfunction
• Allergic dermatitis
• Myofascial pain
• Stimulation tolerance
• Pain over the device
CLINICAL PEARLS AND PITFALLS
• Intimate knowledge of peripheral nerve anatomy is crucial to device placement success and reducing patient morbidity and mortality.
• Image guidance is mandatory for percutaneous PNS.
• Patient candidacy for PNS and PNfS centers on treating neuropathic pain, and as with any implantable device, requires psychological prescreening.
• Percutaneous PNS is an off-label use of a device approved for SCS, and therefore requires adaptation of the SCS placement tools.
• Judicious topicalization at the introducer needle’s entry site is required to enable intraoperative PNS and PNfS testing.
• Avoidance of crossing mobile areas is recommended with IPG site location and tunneling.
• The most common complication, like SCS, is lead migration.
• Overall, the morbidity and mortality associated with PNS and PNfS is low.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






