16 Percutaneous Neuromodulation Therapy
How PNT Works
The transmission of pain signals throughout these pathways depends on the balance of neurotransmitters whose release depends, in turn, on a variety of factors, including the frequency of stimulation of the peripheral nerves. Rather than reviewing the various forms of gate theory, originally proposed by Melzack and Wall,1 we will focus here on the influence of different frequencies of electrostimulation on neurotransmitter release in the central nervous system because this neuromodulation underlies the therapeutic effectiveness of PNT. Much of this knowledge derives from electroacupuncture and neurosurgery research, as noted earlier.
Early work by Han and colleagues from Beijing Medical University demonstrated that analgesia induced by electroacupuncture of different frequencies is mediated by different opioid receptors.2 Analgesia induced by low-frequency (2 Hz) electroacupuncture resulted from activation of mu- and delta-opioid receptors, whereas analgesia induced by high-frequency (100 Hz) electroacupuncture derived from activation of kappa-opioid receptors. Intermediate frequency stimulation (2 to 15 Hz) activated all three types of receptors in the spinal cord of rats.
Further work by Han and colleagues3 showed that low-frequency (2 Hz) peripheral stimulation produces a significant increase in enkephalin release into the lumbar cerebrospinal fluid (CSF), whereas high-frequency (100 Hz) peripheral stimulation increases dynorphin release. This validated earlier work by Mayer and colleagues4 and Pomeranz and Chiu.5 Wang and associates went on to establish that a more potent analgesia could be established by asynchronous electroacupuncture stimulation—alternating between low-frequency (2 Hz) and high-frequency (100 Hz) stimulation—than could be attained by synchronous electroacupuncture stimulation, combining low and high-frequency stimulation at the same time.6 The clinical application of these findings will become evident later in this chapter.
The endorphins (enkephalin and dynorphin) are only part of an ever-elaborating story of pain neurotransmission. In the last few years, research has suggested a role in electroacupuncture (and, thus, PNT) for cocaine and amphetamine-regulated transcript (CART) peptide,7 arginine vasopressin,8 serotonin, catecholamine, and spinal Fos expression,9 interleukin-1-beta,10 corticotrophin-releasing hormone.11 No doubt more neurotransmitters and neurochemicals will play important parts in the transmission and perception of pain.
The PNT Technique
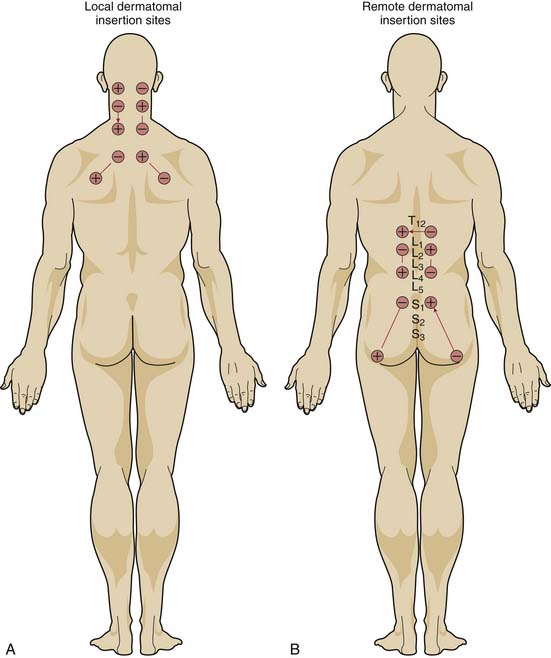
Location of Needles
If the mechanism for analgesia relies primarily on increases in analgesic-like neurotransmitters within the central nervous system induced by peripheral nerve stimulation, varying the level of spinal stimulation should yield similar analgesic effects. Alternatively, if central neuromodulatory changes are primarily responsible for the analgesic effects, then stimulation of peripheral nerves in or near the affected area should prove more effective than stimulation of distant peripheral nerves. White, Craig, and others clarified the effect of the location of electrical stimulation on the acute analgesic response to PNT in a crossover study of 68 patients with nonradiating neck pain.12
When PNT was applied to needles located in the dermatomal distribution of the neck pain, visual analog pain scores decreased significantly more than when PNT was applied to needles located in the low back region or when needles were inserted without electrical stimulation. PNT applied to local needles also brought significant improvements in physical activity, quality of sleep, psychological well-being, and the need for oral analgesic medications, compared with PNT applied to distant needles or unstimulated needles. Moreover, unlike the other two treatments, PNT with the local needles showed a cumulative improvement over the course of therapy (Fig. 16-1).
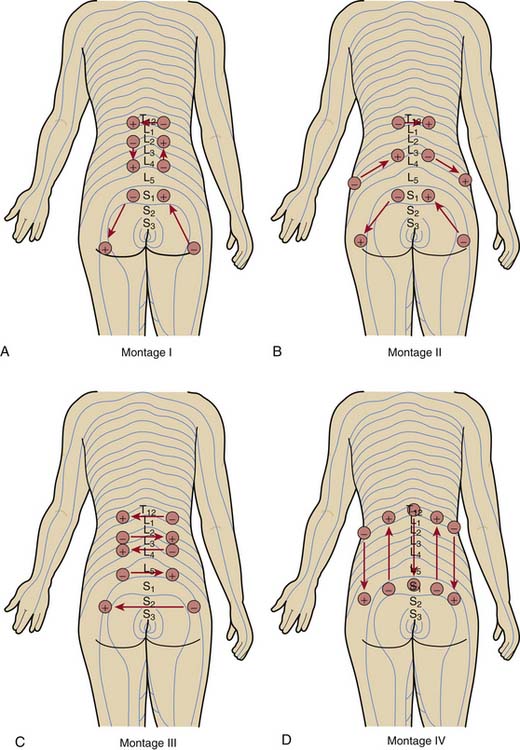
Stimulation Montage
Having placed the needles into the appropriate dermatomes, does it matter how one connects the stimulator leads? In other words, does one specific pattern of electrical stimulation (i.e., montage) provide greater relief than another specific pattern? Work by White and associates demonstrates that montage matters.13
The investigators evaluated the effect of four different montages on the acute analgesic response to PNT when applied at the same dermatomal levels in a crossover study of 72 patients with low back pain (Fig. 16-2). Although all four montages provided significant improvements in pain visual analog scores and in the physical component summary (PCS) and the mental component summary (MCS) of the SF-36, one montage—in which the flow of electrical stimulation paralleled the dermatomes on both sides—produced significantly greater improvements. The cumulative effects over the course of 2 weeks of treatment were also superior with this montage than with the other patterns.
Stimulus Frequency
From the animal and human data already described, it would appear that stimulus with alternating frequencies should afford greater pain relief than stimulus with a single frequency. Ghoname and colleagues tested this hypothesis by comparing the effect of three different frequencies on the analgesic response to PNT therapy in 68 patients with low back pain resulting from degenerative lumbar disc disease. Patients were treated with PNT therapy at 4 Hz, alternating 3-second pulses of 15 Hz and 30 Hz, and 100 Hz, as well as sham-PNT (0 Hz), in random order, three times weekly for 2 weeks, with 1 week off between each sequence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree