Intracranial physiology
 Pediatric anesthetic requirements
Pediatric anesthetic requirements
 The pediatric airway and pulmonary physiology
The pediatric airway and pulmonary physiology
 Anesthetic considerations
Anesthetic considerations
 Neuroanesthetic management for special procedures
Neuroanesthetic management for special procedures
 Hydrocephalus
Hydrocephalus
 Craniosynostosis
Craniosynostosis
 Intracranial tumors
Intracranial tumors
 Surgery for epilepsy
Surgery for epilepsy
 Head trauma
Head trauma
 Meningomyelocele and encephalocele
Meningomyelocele and encephalocele
 Craniofacial surgery
Craniofacial surgery
 Vascular anomalies
Vascular anomalies
 Neuroradiology
Neuroradiology
 Anesthetic management of neurodiagnostic and neurointerventional procedures
Anesthetic management of neurodiagnostic and neurointerventional procedures
 Periprocedural neuroradiologic management
Periprocedural neuroradiologic management
I. INTRACRANIAL PHYSIOLOGY
While the development of the central nervous system (CNS) is incomplete at birth, maturation continues until the end of the first year of life. During this period, dramatic changes in intracranial parameters can occur primarily due to growth in brain tissue, changes in cerebral blood volume (CBV) and cerebrospinal fluid (CSF) production, as well as the closure of skull sutures. CBV is directly determined by cerebral blood flow (CBF), which is usually 100 mL/100 g/minute in children from 3 to 12 years of age. The CBF in children from 6 to 40 months is 90 mL/100 g/minute and in newborns and premature infants, it is approximately 40 to 42 mL/100 g/minute. In the absence of CBF or CBF < 6 mL/100 mg/minute, cell death occurs, while CBF of 15 to 20 mL/100 g/minute is associated with significant neuronal dysfunction. Any changes in CBF leading to changes in CBV can cause logarithmic changes in intracranial pressure (ICP) secondary to limited skull compliance. As a result, anesthesiologists must constantly be aware of factors that can cause changes in cerebral perfusion.
In stark contrast to autoregulation of CBF in adults, autoregulation limits in infants and children are largely unknown. However, the limits are thought to be lower in value than those in adults and can be easily impaired or abolished. This can lead to varying degrees of intraventricular hemorrhage (IVH) with grave consequences. Studies have demonstrated that hyperventilation restores autoregulation in the neonate and that CBF velocity changes logarithmically and directly with end-tidal carbon dioxide tension (ETCO2) in infants and children. Under normal conditions, ICP depends more on CBF and CBV than on CSF production. All inhalational anesthetics must, therefore, be used with care because they increase CBF and CBV by directly producing vasodilatation. New advances in continuous monitoring of autoregulation are being researched by analyzing spontaneous slow waves of cerebral perfusion pressures (CPPs), the difference between mean arterial pressure (MAP) and intracranial pressure.
II. PEDIATRIC ANESTHETIC REQUIREMENTS
The anesthetic requirements in pediatric patients vary with age and maturity. Neonates and premature infants have decreased anesthetic requirements relative to older children. The reasons for the lower requirements in babies are the immaturity of the newborn’s nervous system, the presence of maternal progesterone, elevated levels of endorphins, and the immaturity of the blood–brain barrier. Research, over the last 20 years, has reinforced the notion that neonates do sense pain and can develop a stress response to surgical stimulation. They, therefore, require adequate levels of anesthesia to blunt the stress response. Secondary to immature organ systems, neonates are sensitive to anesthetic drugs and therefore may benefit more from a narcotic-based anesthetic. While such an anesthetic technique may offer more hemodynamic stability, emergence may be delayed because the liver and kidneys are not fully developed. However, inhalation or intravenous induction of anesthesia in infants is more rapid than that in adults because of the following reasons:
A. The ratio of alveolar ventilation to functional residual capacity (FRC) is 5:1 in the infant and 1.5:1 in the adult.
B. The neonate has a greater cardiac output per kilogram of body weight than the adult. A neonate’s cardiac output can typically range between 200 and 325 mL/kg/minute.
C. More of the neonate’s cardiac output goes to the vessel-rich group of organs including the brain (up to 25%) and the heart.
D. The infant has a lower blood–gas partition coefficient for volatile anesthetics and a lower anesthetic requirement.
1. The rapid induction of anesthesia occurring with most volatile anesthetics may be hazardous in the premature, small for gestational age, or unstable patient.
2. Whatever the choice of anesthetic drugs, sick neonates require resuscitation and normalization of fluid and electrolyte balance before the induction of anesthesia.
The common denominator of neonatal surgery is that the operations are frequently performed emergently. This contributes significantly to the greater than tenfold increase in perioperative morbidity and mortality in neonates as compared to other pediatric age groups. Additional difficulties can arise because intraoperative hypoxia and hemodynamic instability can be the first indication of previously unrecognized congenital cardiac and pulmonary anomalies. Regression to fetal circulation, leading to increases in pulmonary artery pressures, may also occur intraoperatively in neonates because of hypercarbia, hypoxia, hypothermia, and acidosis. In addition, respiratory complications are not uncommon in neonates owing to the small size of their airway, congenital laryngotracheal lesions, craniofacial anomalies, and acute (e.g., respiratory distress syndrome [RDS], transient tachypnea of the newborn) or chronic (e.g., bronchopulmonary dysplasia) lung disorders.
III. THE PEDIATRIC AIRWAY AND PULMONARY PHYSIOLOGY
The neonatal period is considered as the first 30 days of extrauterine life and includes the newborn period, defined as the first 24 hours of life. Up to 3 to 5 months of age, the infant is an obligate nose breather in part because of the immaturity in the coordination between respiratory efforts and the oropharyngeal motor and sensory input. As a result of being an obligate nose breather, conditions such as congenital choanal atresia or simple nasal congestion can cause respiratory distress and asphyxia in the infant. Pediatric patients also have anatomically different airways than adults. Along with the cricoid cartilage’s being the narrowest part of their trachea, infants have a narrower and angled epiglottis. Additionally, an infant has a larger occiput and a proportionally larger tongue. All of these factors can make the trachea of an infant or child more difficult to intubate.
Furthermore, infants are more prone to rapid deoxygenation. Oxygen consumption in the infant is high—7 to 9 mL/kg as compared to 3 mL/kg in the mature state. Infants also have a high closing volume leading to early alveolar collapse and a high minute ventilation-to-FRC ratio. As a result, pediatric anatomy and pulmonary physiology make airway management more difficult and critical.
IV. ANESTHETIC CONSIDERATIONS
A. Preoperative assessment
The preoperative assessment of a pediatric patient who has neurologic dysfunction involves establishing the degree of change in the cerebral compliance. The clinical presentation varies with the age of the patient as well as the rapidity and degree of change in the intracranial contents. Infants might present with a history of irritability, lethargy, and failure to feed. They may have an enlarging head circumference or bulging fontanelle. Older children might present with headaches, nausea, vomiting, or change in the level of alertness. Funduscopic examination may reveal papilledema although this may be a late sign in neonates owing to the presence of an open fontanelle. Some children, especially neonates, might need further evaluation by pediatric cardiologists and other subspecialists because of the presence of congenital cardiopulmonary disorders and other coexisting diseases.
B. Fluid balance
Any evaluation must also include assessing pre-existing fluid and electrolyte imbalances from lack of oral intake or active vomiting secondary to changes in the ICP. Furthermore, fluid restriction and the combination of hyperosmolar (e.g., mannitol) and diuretic (e.g., furosemide) therapy may result in hemodynamic instability and shock when coupled with intraoperative blood loss. It is, therefore, necessary to establish and maintain normovolemia throughout the perioperative period. While normal saline (or other non-glucose-containing solutions) may be used as the maintenance fluid because it is slightly hyperosmolar to plasma, enough glucose must also be administered to prevent hypoglycemia. It is imperative to secure excellent intravenous access for fluid and blood replacement and drug delivery before the start of the operation because the opportunities to do so will be limited once the operation is in progress. Two large-bore intravenous catheters, an arterial catheter, and a urinary catheter are necessary for children undergoing craniotomy, craniofacial reconstruction, or extensive spine procedures.
C. Administration of glucose
The administration of glucose-containing fluids during neurosurgical procedures is determined by the intraoperative measurement of blood glucose. The automatic addition of glucose to intraoperative maintenance fluid is unnecessary because hypoglycemia may not be a common occurrence in fasting pediatric patients, even in infants < 1 year of age. This is especially true because the stress response to surgery itself results in hyperglycemia from increased sympathoadrenal activity with decreased glucose tolerance, decreased glucose utilization, and increased gluconeogenesis. Furthermore, hyperglycemia, as defined by serum glucose ≥ 200 mg/dL, is associated with higher infection, neuronal loss, and higher mortality rates and is particularly critical in pediatric patients at risk for hypoxic–ischemic insults.
Solutions containing 1% to 2.5% glucose are less likely to cause hyperglycemia than are 5% solutions, especially when administered at a rate of 120 mg/kg/hour (2 to 5 mg/kg/minute). This is sufficient to maintain an acceptable blood glucose level and prevent lipid mobilization in infants and children. The monitoring of intraoperative blood glucose and the continual adjustment of glucose administration may be necessary in certain circumstances (Table 15.1).
The rate of delivery of hyperalimentation may need to be reduced intraoperatively owing to a decrease in the glucose requirement of children receiving hyperalimentation. Alternatively, patients may receive a continuous infusion of dextrose 10% in water (D10W), but will still require intraoperative glucose monitoring.
D. Premedication
Sedative premedication should be avoided in all patients suspected of increases in ICP because these drugs may further reduce the respiratory drive, leading to hypercarbia, cerebral vasodilatation, and, ultimately, tonsillar herniation. Patients with normal ICPs scheduled for the repair of vascular lesions may be sedated to control preoperative anxiety and avoid hypertension and rupture of the vascular abnormality.
TABLE 15.1 Intraoperative Glucose Monitoring and Administration of Glucose
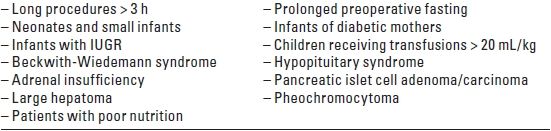
IUGR, intrauterine growth retardation.
E. Inhalational and intravenous anesthetics
Inhalational anesthetics affect mean arterial pressure (MAP), ICP, and CPP in children. At 0.5 and 1 minimum alveolar concentration (MAC), sevoflurane, isoflurane, and desflurane with 60% nitrous oxide (N2O) increase ICP and decrease MAP and CPP in a dose-dependent manner. There is no relationship between the patient’s baseline ICP and the ICP elevation after exposure to sevoflurane and isoflurane. Desflurane and isoflurane, however, may increase ICP to a greater extent than halothane or sevoflurane in children whose ICP is elevated preoperatively. The gradual increase in ICP from desflurane may be secondary to an increase in CSF production or a decrease in CSF absorption. Because the effect of a change in MAP on CPP is three to four times greater than the effect of a change in ICP, maintaining MAP is the more important factor in preserving CPP. For children who have a known increase in ICP, intravenous anesthesia may be the better alternative.
With propofol and thiopental administration, there is a dose-dependent decrease in CMRO2, CBF, and ICP until an isoelectric encephalogram (EEG) is achieved. These effects have been noted within 2 minutes after administration and may, thereby, provide a safe alternative to volatile drugs for reducing ICP.
Dexmedetomidine, an alpha-adrenergic agonist similar to clonidine, is also being used with increasing frequency for the sedation of pediatric patients. Its hemodynamically-stable profile and lack of respiratory depression make dexmedetomidine a good choice for children who require ongoing sedation in the perioperative period. While its effects on CMRO2, CBF, and ICP are still being investigated, CMRO2 –CBF coupling appears to be preserved.
Opioid analgesics, such as fentanyl and sufentanil, have not been shown to cause an increase in ICP in the setting of controlled ventilation. These specific opioids also have the additional benefit of a short duration of action, thereby allowing for rapid neurologic assessment upon emergence from anesthesia.
Succinylcholine’s effect on raising ICP has been extensively documented in the literature and is, in part, due to muscle fasciculations and the resultant increase in intra-abdominal and intrathoracic pressures. This elevation in ICP is seen even in babies, who do not typically fasciculate due to low muscle tone. It is, therefore, contraindicated as a muscle relaxant in the setting of elevated ICP. Conversely, a rapid-sequence induction dose of 1 to 1.2 mg/kg of rocuronium has not been shown to have any deleterious effects on ICP.
F. Monitoring
Monitoring depends upon the patient’s age and condition and the planned surgical procedure. Routine monitoring includes the use of the precordial stethoscope, electrocardiogram (ECG), oxygen saturation (SpO2) by pulse oximeter, ETCO2, non-invasive blood pressure (NIBP) measurement, temperature probe, and a peripheral nerve stimulator to monitor the degree of neuromuscular blockade. Direct arterial blood pressure monitoring, at least two good peripheral intravenous catheters, and a urinary catheter are recommended for extensive and invasive surgical procedures. Measurement of central venous pressure (CVP) may not reflect intravascular volume accurately, especially in patients in the prone position. As a result, unless a CVP is being inserted for management of intraoperative venous air embolism or for additional intravenous access, the risk of inserting a CVP catheter may exceed the benefits.
G. Venous air embolism (VAE)
VAE occurs commonly during craniotomy in infants because of the head position and surgical approach. The head of a small child, particularly the occiput, is large in relation to the rest of the body, causing it to lie above the heart, even in the supine position. In addition, the head of the bed is often elevated to facilitate drainage of blood and CSF during the operative period. Pressure within the superior sagittal sinus decreases as the head is elevated, increasing the likelihood of negative intravenous pressure and entrainment of air, thereby resulting in a VAE. Patients who have a patent ductus arteriosus or foramen ovale are also at risk for paradoxical air embolism through these defects. Consequently, precordial Doppler ultrasonography is used in conjunction with ETCO2 sampling for detecting and assessing the treatment of VAE. The optimal position for the Doppler probe is on the anterior chest just to the right of the sternum in the fourth intercostal rib space. Alternatively, the probe may be positioned on the posterior chest in infants weighing < 6 kg. The anesthesiologist may also elect to monitor for the presence of nitrogen in the end-tidal gas mixture. Due to the fact that VAE has occurred in the sitting, prone, and supine positions, the use of N2O should be avoided to prevent an increase in the size of entrained air bubbles.
H. Neurophysiologic monitoring
Monitoring of evoked potentials, electrocorticography, and EEG necessitates low concentrations of inhalational anesthetics. Inhalational anesthetics and N2O depress somatosensory evoked potentials (SSEPs) for operations on the spine and brain stem; a total intravenous anesthetic (TIVA) technique using narcotics and a propofol infusion may be preferable. The use of electromyography and motor evoked potentials (MEPs) requires that muscle relaxation be reversed and that minimal amounts of volatile inhalational anesthetics are used during electromyography and monitoring of muscle movement.
I. ICP monitoring
ICP monitoring has seen increased utilization in the management of pediatric head injury because it facilitates intraoperative and postoperative assessment of a patient’s response to therapies. Normal ICP is 0 to 10 mm Hg and can increase due to mass effect, increased CSF, tissue edema, or intracranial bleeding. ICP after traumatic brain injury is controlled by adjusting CPP and the partial pressure of arterial carbon dioxide (PaCO2) via minute ventilation adjustments. Surgeons may also request that steroids, mannitol, and furosemide be administered as a means of decreasing intracranial volume. Microcirculation around contusions is enhanced by maintaining normovolemia and decreasing sympathetic discharge by maintaining adequate levels of anesthesia. This approach has been correlated with an improvement in outcome from traumatic brain injury over the past 10 years.
J. Temperature regulation
Hypothermia, an often overlooked issue in infants and small children, usually requires active heating in the operating room by elevating room temperature, using warm-air blankets, radiant warming lights, and humidification of inspired gases, and warming intravenous fluids. However, recent research with pediatric patients suggests that treatment with moderate hypothermia (32°C to 33°C) up to 24 hours after initial injury may decrease mortality.
K. Positioning
The extended duration of neurosurgical procedures and the unusual access requirements necessitate paying close attention to the positioning of the patient before placing surgical drapes. This can be achieved through the use of careful padding of potential pressure points, checking peripheral pulses, and avoiding stretching of peripheral nerves. For patients operated on in the prone position, there must be free movement of the abdominal wall without undue flexion of the head. Excessive neck flexion may cause the endotracheal tube to kink, exert excessive pressure on the tongue, or advance the tube into a mainstem bronchus. The resultant hypoxia and hypercarbia will increase ICP, potentially leading to upper spinal cord and lower brain stem ischemia. Patients who already have posterior fossa abnormalities such as a mass lesion or Arnold-Chiari malformation are especially at risk for this complication. Pediatric patients may also experience flexion-induced swelling of the head and tongue from obstruction of venous and lymphatic drainage. This may result in postextubation obstruction of the airway or croup. Extreme rotation of the head can further limit venous return through the jugular veins, leading to increased ICP, impaired cerebral perfusion, and bleeding from cerebral veins.
L. Emergence
The goals for emergence include prompt awakening to aid early assessment of neurologic function, hemodynamic stability, and minimal coughing or straining on the endotracheal tube to avoid intracranial hypertension and bleeding. Patients may receive fentanyl before emergence; arterial hypertension is treated with drugs such as esmolol, labetalol, and hydralazine. Naloxone is avoided because its use has been associated with uncontrolled hypertension and coughing when the endotracheal tube is in place.
The trachea is extubated after the patient responds to commands or when infants and toddlers open their eyes. Alternatively, some anesthesiologists prefer to extubate the trachea when the patient is still deeply anesthetized as long as there are no contraindications (e.g., intraoperative catastrophe, loss of airway reflexes, poor preoperative condition, class III or IV airways). If the patient’s awakening is delayed and no anesthetic cause can be determined, the presence of an acute neurologic issue should be ruled out by a computed tomography (CT) scan before tracheal extubation.
M. Postoperative intubation
There are several circumstances in which the trachea should remain intubated into the postoperative period. Operations that interfere with cranial nerve nuclei or brain stem function, with resultant impairment of airway reflexes and respiratory drive, require ongoing airway protection and ventilation until these functions can be assessed. The loss of several blood volumes, even with replacement, may necessitate continued maintenance of an artificial airway and protection of the airway reflexes due to fluid shifts and possible laryngeal edema. Also, prolonged operation in the prone position may lead to edema of the face and airway with the possibility of airway obstruction after extubation. If dexmedetomidine has been used in the intraoperative period with an initial loading dose of 1 to 2 mcg/kg over 10 minutes and a continuous infusion of 0.2 to 0.7 mcg/kg/hour, the infusion can be continued at the same rate in the postoperative period for up to 24 hours as a means of maintaining sedation. Of note is the fact that the rapid administration of dexmedetomidine may increase pulmonary artery pressure and pulmonary vascular resistance.
N. Postoperative care
Complications in the postoperative period involve a number of organ systems. Respiratory dysfunction in the form of apnea or irregular breathing occurs frequently after posterior fossa craniectomy leading to prolonged postoperative intubation. There may also be airway obstruction secondary to either edema or cranial nerve injury. Operative injury to either the hypothalamus or the pituitary gland can lead to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) or diabetes insipidus (DI) manifesting as seizures, changes in the level of consciousness, and abnormalities of fluid and electrolyte (especially sodium) balance. When children require sedation for endotracheal intubation postoperatively, the administration of propofol is not recommended for long-term sedation due to reports of children who have developed metabolic acidosis, lactic academia, and bradyarrhythmias after prolonged infusion. Avoidance of propofol infusion syndrome can likely be achieved by limiting infusions to < 48 hours in duration with infusion rates less than approximately 70 mcg/kg/minute and with monitoring for acid/base disturbances.
V. NEUROANESTHETIC MANAGEMENT FOR SPECIAL PROCEDURES
A. Hydrocephalus
1. Etiology
Hydrocephalus is caused by the enlargement of the ventricles from increased production of CSF, decreased absorption of CSF by the arachnoid villi, or obstruction of the CSF pathways. It is often manifested as an increase in the head circumference in pediatric patients. Hydrocephalus is typically classified as communicating/non-obstructive or non-communicating/obstructive (see Table 15.2). The causes of increased CSF collection can be congenital or acquired.
2. Preoperative management
The patient should be assessed for any effects of elevated ICP such as nausea or vomiting, changes in the ventilatory pattern, irritability, decreased level of consciousness, bradycardia, or hypertension. Further, obtaining a CT scan may demonstrate increases in the size of the ventricles. Sudden neurologic deterioration in the pediatric patient must be treated quickly with emergent endotracheal intubation, muscle relaxation, hyperventilation with ETCO2 monitoring, and administration of drugs to reduce cerebral metabolic demands (e.g., barbiturates) and diuretics (e.g., mannitol and furosemide) until emergency surgical reduction of the ICP is achieved. In rare circumstances, control of ICP is accomplished by direct needle puncture of the lateral ventricle and aspiration of CSF or by emergency bedside placement of CSF drains.
TABLE 15.2 Causes of Hydrocephalus
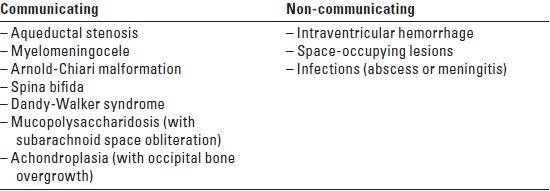
3. Premedication
Sedation is relatively contraindicated in patients who have hydrocephalus because the resulting hypoventilation may increase ICP. EMLA cream (a eutectic mixture of local a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






