Pediatric Intestinal and Multivisceral Transplantations
Geoffrey J. Bond
Kathryn A. Felmet
Ronald Jaffe
Kyle A. Soltys
Jeffrey A. Rudolph
Dolly Martin
Rakesh Sindhi
George V. Mazariegos
KEY POINTS
 Causes of irreversible intestinal failure in children are predominantly due to surgical conditions (mostly short gut syndrome from volvulus, necrotizing enterocolitis, gastroschisis, etc.). In addition, medical conditions can lead to functional intestinal failure, such as motility disorders (e.g., intestinal pseudo-obstruction), and enterocyte dysfunction, such as microvillus inclusion disease.
Causes of irreversible intestinal failure in children are predominantly due to surgical conditions (mostly short gut syndrome from volvulus, necrotizing enterocolitis, gastroschisis, etc.). In addition, medical conditions can lead to functional intestinal failure, such as motility disorders (e.g., intestinal pseudo-obstruction), and enterocyte dysfunction, such as microvillus inclusion disease. Indications for intestinal transplantation include (a) evidence of liver dysfunction or failure, (b) loss of major venous access, (c) frequent central line-related sepsis, and (d) recurrent episodes of severe dehydration despite intravenous fluid management.
Indications for intestinal transplantation include (a) evidence of liver dysfunction or failure, (b) loss of major venous access, (c) frequent central line-related sepsis, and (d) recurrent episodes of severe dehydration despite intravenous fluid management. Recipient operations should be tailored to the specific indications for each patient and include isolated intestinal transplantation, combined liver and intestinal transplantation, and multivisceral transplantation including the stomach (with or without the liver).
Recipient operations should be tailored to the specific indications for each patient and include isolated intestinal transplantation, combined liver and intestinal transplantation, and multivisceral transplantation including the stomach (with or without the liver). Immunosuppression for intestinal transplantation is based on tacrolimus and antibody induction therapy.
Immunosuppression for intestinal transplantation is based on tacrolimus and antibody induction therapy. Current modifications in intestinal transplantation include pretreatment of the recipient with an antilymphocyte antibody such as either antithymocyte antibody or basiliximab to eliminate maintenance steroid use postoperatively.
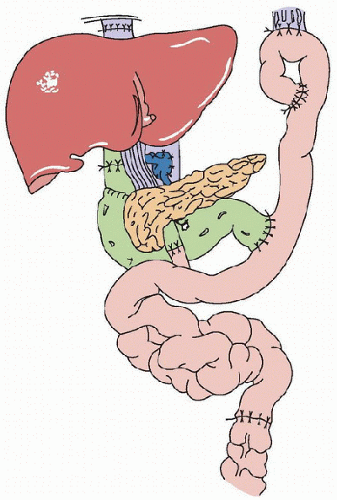
Current modifications in intestinal transplantation include pretreatment of the recipient with an antilymphocyte antibody such as either antithymocyte antibody or basiliximab to eliminate maintenance steroid use postoperatively.Intestine was one of the first organs to be transplanted (1), but early case reports noted a high incidence of graft loss from rejection, infection, and technical complications (2). In 1987, a 3-year-old girl received a multivisceral abdominal graft that included the stomach, duodenum, pancreas, small bowel, colon, and liver and survived with good intestinal graft function for 6 months (3). A modification of this operation was reported in 1989 that involved transplantation of a “cluster” of organs consisting of the liver and the pancreaticoduodenal complex (Fig. 104.1) (4). Unfortunately, until 1990, there were only two survivors of isolated cadaveric intestinal grafts (5,6). In 1989, the introduction of the immunosuppressive agent tacrolimus (FK506, Prograf) revolutionized the field by permitting successful transplantation of human intestinal grafts (alone or as part of a multivisceral graft) (7).
INDICATIONS
Diseases associated with loss of intestinal function and the need for transplantation can be divided into surgical and nonsurgical etiologies. Patients with surgical causes generally suffer from loss of bowel length after resections due to ischemia, obstruction, or from strictures and fistulas (as with Crohn disease). With nonsurgical causes of intestinal failure, the anatomic length and gross morphology of the intestine are often normal. Nonsurgical causes of intestinal failure include motility disorders (e.g., intestinal pseudo-obstruction, Hirschsprung disease) (8,9) and disorders of enterocyte function (e.g., microvillus  inclusion disease) (10). Table 104.1 lists the indications for transplantation in the case experience at the University of Pittsburgh and Children’s Hospital of Pittsburgh.
inclusion disease) (10). Table 104.1 lists the indications for transplantation in the case experience at the University of Pittsburgh and Children’s Hospital of Pittsburgh.
 inclusion disease) (10). Table 104.1 lists the indications for transplantation in the case experience at the University of Pittsburgh and Children’s Hospital of Pittsburgh.
inclusion disease) (10). Table 104.1 lists the indications for transplantation in the case experience at the University of Pittsburgh and Children’s Hospital of Pittsburgh.Parenteral nutrition (PN) is the standard of care for patients with intestinal failure who are unable to maintain a normal nutritional, fluid balance and electrolyte state (11,12). The management of intestinal failure should be multidisciplinary and provide a medical focus to optimize nutritional status while minimizing cholestatic liver injury and a surgical focus to consider options (such as stoma closure or bowel lengthening) that reduce the need for total PN (TPN) and, ultimately, transplantation (13,14). A group of patients persist who develop irreversible intestinal failure and suffer from complications of indefinite PN therapy. Transplantation of the intestine either alone or accompanied by other intra-abdominal organs (liver, stomach, duodenum, pancreas) may be lifesaving for these patients (15).
Decisions regarding the best transplant options are based on the anatomic and functional integrity of the remaining gut and abdominal organs as well as their vascular supplies. Liver replacement in intestinal transplant candidates is based on biochemical dysfunction (hyperbilirubinemia, transaminase abnormalities, hypoalbuminemia, thrombocytopenia, and coagulopathy), the presence of bridging fibrosis or cirrhosis on liver biopsy, and the presence of portal hypertension. Hypercoagulable patients deficient in protein S, protein C, and antithrombin III (16) may develop diffuse thromboses within the splanchnic system and undergo transplantation for mesenteric venous hypertension rather than for intestinal failure. Unfortunately, liver transplantation may not be technically feasible because of extensive portomesenteric thrombosis in some patients.
In October 2000, the Center for Medicare and Medicaid Services (CMS) in the United States approved intestinal, combined liver and intestinal, and multivisceral transplantation (17,18) at centers of excellence as a standard of care for patients with irreversible intestinal failure who could no longer be maintained on PN because of (a) impending liver failure, as manifested by jaundice or elevated liver injury tests, clinical findings (splenomegaly, varices, coagulopathy), history of stomal bleeding, or hepatic cirrhosis on biopsy; (b) loss of major venous access defined as more than two thromboses in the great vessels (subclavian, jugular, and femoral veins); (c) frequent central line-related sepsis consisting of more than two episodes of systemic sepsis per year or one episode of line-related fungemia associated with septic shock or acute respiratory distress syndrome; or (d) recurrent episodes of severe dehydration despite intravenous fluid  management.
management.
 management.
management.TABLE 104.1 INDICATIONS FOR COMPOSITE AND ISOLATED INTESTINAL TRANSPLANTATION | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CLINICAL MANAGEMENT OF THE PRETRANSPLANT CHILD IN THE PICU
Intensive care of children with intestinal or combined intestinal and liver failure presents a unique set of challenges that fall into two categories: (a) PN dependence and the associated vascular access issues, and (b) the development of liver disease. PN dependence and the long-term requirement for central access bring a set of problems (discussed in detail in the next section). Liver disease develops in 40%-60% of infants who require long-term PN for intestinal failure, and although it may not ultimately require liver transplant, it can complicate their management (also discussed in detail in a later section) (19). Both problems are compounded by the long wait times before size-appropriate organs are available (often 6 months to 1 year), and they make it important for potential intestinal transplant candidates to be referred to a transplant service for evaluation as soon as possible. The success of the intestinal or multivisceral transplantation depends in large measure on the health and nutritional status of the transplant recipient. Even if the child does not ultimately require a transplant, early referral to a center experienced in the management of intestinal failure, including surgical and transplantation options, can dramatically impact outcomes.
The goal of critical care is to keep the child in optimal condition to receive a transplant. For this reason, the intensivist should be in close communication with the transplant surgeon both to review the patient’s status and to discuss PICU interventions that may interfere with the patient’s candidacy.
Parenteral Nutrition Dependence
PN-dependent patients have chronic problems with venous access because of infection and thrombosis. Any patient with a central line has increased susceptibility to infection, but intestinal transplant candidates are unique in several ways. Prevention of infection is particularly important since episodes of sepsis contribute to the deterioration in liver function (20,21). The central lines of patients with short gut syndrome can become infected by external contamination of the line or by translocation of bacteria across a gut with inadequate barrier function (22). Because patients have been repeatedly exposed to broad-spectrum antibiotics, and because intestinal stasis contributes to bacterial overgrowth, infections that result from bowel translocation may be multidrug resistant (23). The risk of fungal infections is also increased in this patient group compared to the general population (22). Enteric feedings may help preserve the intestinal mucosal barrier function and decrease infectious complications (23).
Patients with liver failure have impaired immune responses. The oxidative burst function of neutrophil and Kupffer cells is impaired and complement levels are reduced (22,24).
Additionally, adrenal insufficiency with failure of the stress cortisol response is common in patients with acute and chronic liver disease (25,26). The combination of adrenal insufficiency, relative immune deficiency, and the intestinal transplant candidate’s requirement for invasive procedures creates a dangerous susceptibility to severe sepsis and septic shock.
Additionally, adrenal insufficiency with failure of the stress cortisol response is common in patients with acute and chronic liver disease (25,26). The combination of adrenal insufficiency, relative immune deficiency, and the intestinal transplant candidate’s requirement for invasive procedures creates a dangerous susceptibility to severe sepsis and septic shock.
Treatment for septic shock should follow established parameters (27) with a few caveats. The cardiac function of intestinal transplant candidates with liver disease is not normal. Patients with severe liver failure may have a hyperdynamic state at baseline, but a blunted contractile response to stress. Both systolic and diastolic ventricular function may be impaired (28). Clinical experience indicates that children with cirrhotic cardiomyopathy may not tolerate large-volume fluid challenge. Resuscitation should be appropriately aggressive, but with careful attention to signs of intravascular volume overload as well as vigilance for concomitant hepatopulmonary or hepatorenal syndrome. Palpation of the liver may not be a reliable indicator of intravascular volume overload in patients with cirrhosis; instead, clinicians should investigate changes in central venous pressure, development of rales on lung exam, and changes on chest x-ray. Echocardiogram can be used in difficult cases to assess cardiac filling and function. In patients with advanced liver disease, albumin may be preferable to crystalloid as a resuscitation fluid to avoid worsening anasarca. High salt loads (e.g., normal saline) should be avoided in established liver disease.
Inotropic or vasopressor agents should be used if clinical signs suggest the child is intravascularly replete. Low diastolic blood pressures may be present in association with advanced liver disease (28,29). Early septic shock in these children follows a vasodilatory pattern commonly seen in adults and may respond to vasopressor agents. In patients with catecholamine unresponsive septic shock, adrenal function should be evaluated with a cortisol level and/or adrenocorticotropic hormone (ACTH) stimulation test. In some cases, it may be appropriate to treat adrenal insufficiency empirically (hydrocortisone bolus and intermittent dosing or continuous infusion) while results of these tests are pending.
When infections cannot be cleared, it may be necessary to remove and replace lines. As children awaiting intestinal transplant are dependent on central access, meticulous care must be given to prevention of infection and preservation of line sites when possible. Intestinal transplant candidates are at high risk for forming clots around central lines that can become occlusive and persist after line removal. An inadequate synthesis of both coagulation factors and anticoagulating factors (e.g., protein C, protein S, and antithrombin III) is common in patients with liver failure (22). The loss of two or more great vessels to thrombosis is part of the criteria for considering transplantation, but clotting in most or all of the available sites for central venous catheterization can make transplantation technically challenging or impossible. When new lines are being placed, care should be taken not to damage the vessel unduly. Before line placement a careful clot history should be taken, and when appropriate, Doppler ultrasound of great vessels should be used to guide the individual performing the procedure. In some cases, especially older children, venograms of the extremities are performed as part of the pretransplant workup to better delineate vascular patency that is not accurately assessed by ultrasound (30,31).
A full discussion of nutritional requirements of children with intestinal failure is beyond the scope of this chapter, but it is important to note (a) that the ability to heal after a major operation is partly dependent on preoperative nutritional status, and (b) that the development of liver disease may be influenced by nutritional and metabolic parameters (19,32). A nutritional specialist should routinely be involved in the care of all prospective intestinal transplant patient regardless of location. Whether that care occurs at home or in the hospital, attention should be paid to the provision of PN with adequate calories and an appropriate balance of fats, protein, and carbohydrate calories with an appropriate complement of trace elements and minerals.
Adequate nutrition is crucial, but our clinical experience with preoperative intestinal transplant patients suggests that these patients have a tendency to gain excess adipose tissue. When calculating metabolic requirements of these children in the ICU, it is important to include an estimate of their decreased activity on actual caloric expenditure. When possible, delivery of some enteral calories may preserve intestinal epithelial barrier function, decrease the risk or severity of intestinal failure-associated liver disease, and improve hospital mortality (19,33,34).
Liver Failure
Isolated intestinal transplant candidates may have mild, reversible liver disease. Patients awaiting combined liver and small intestinal or multivisceral transplants may persistently struggle with all of the problems seen in hepatic failure over a waiting period that is long compared to isolated liver candidates. Coagulopathy, portal hypertension with ascites and hepatomegaly, variceal bleeding, hypoalbuminemia, hyperbilirubinemia, hyperammonemia with hepatic encephalopathy, and hepatorenal and hepatopulmonary syndrome may all be seen in this patient population. The effects of a long duration of increased abdominal girth, renal dysfunction, and persistent coagulopathy on patients waiting for combined transplants deserve further discussion.
Intestinal transplant candidates with cirrhosis and liver failure have increased abdominal girth due to organomegaly and ascites. In children, especially infants, the enlarged liver and other abdominal contents may impinge on lung volumes and impede respiration. Although the problem is easily overcome with positive pressure ventilation, mechanical ventilation should be considered a last resort in view of the associated problems of sedation, respiratory muscle deconditioning, and ventilator-associated pneumonia. Due to risk of bleeding and infection, tracheostomy is usually contraindicated and should not be performed without consultation with the transplant surgeon. If ascites predominates over organomegaly as a cause of increased abdominal girth, drainage of ascitic fluid may relieve symptoms. Relief is usually temporary, as the circumstances leading to the fluid collection persist. The indications for peritoneal drainage must be weighed against the risk of infection. Additionally, rapid drainage of large volumes of peritoneal fluid may lead to intravascular hypovolemia and shock.
Patients with combined liver and intestinal or multivisceral transplants often have some degree of renal dysfunction that renders them sensitive to fluid overload. Repeated infections with fungus and gram-negative organisms expose these patients to multiple nephrotoxic agents. Additionally, episodes of septic shock can expose the kidney to low-flow states, causing acute tubular necrosis. Hepatorenal syndrome is a very late finding in liver failure but can contribute to renal insufficiency.
Persistent coagulopathy in combined liver and intestinal transplant candidates is a significant complication. Coagulopathy may be managed with administration of clotting factors. It may be impossible to normalize laboratory indicators of clotting function; instead, clinical evidence of bleeding should guide therapy. Intracranial hemorrhage, though rare, can occur. Correction of disordered coagulation with large volumes of clotting factors can lead to fluid overload. Renal insufficiency associated with exposure to nephrotoxic agents can exacerbate this predisposition to fluid overload. In cases of severe or recurrent bleeding, plasma exchange (plasmapheresis) and judicious use
of recombinant factor 7 have been used successfully to correct coagulopathy without fluid overload (35



of recombinant factor 7 have been used successfully to correct coagulopathy without fluid overload (35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree