Fig. 11.1
A hypothesized multidimensional biobehavioral model of pediatric pain developed to account for the complex and individual pediatric chronic pain experience. Adapted from Varni et al. (1996b), p. 180. © 1996 by Plenum Publishing. Reprinted with permission
This theoretical framework is further delineated into pain antecedents, which have a causal role in pain onset or exacerbate pain intensity, pain concomitants (e.g., depression, anxiety), which occur only during a painful episode and which may be reciprocal, and pain consequences, which persist beyond pain relief and include long-term psychological, social, and physical disability (p. 516).
The validity of this multidimensional Biobehavioral Model of Pediatric Pain was examined in children and adolescents with symptomatic juvenile idiopathic arthritis (JIA) and a variety of other pediatric rheumatic diseases (Sawyer et al. 2004, 2005; von Weiss et al. 2002). A strong negative relationship was observed between children’s self-reported pain intensity with JIA and their self-reported health-related quality of life, including various aspects of functional status (Sawyer et al. 2005). In contrast, effective pain coping strategies in children with JIA have a significant positive effect on the physical, emotional, and social functioning domains that comprise their health-related quality of life (Sawyer et al. 2004). Fewer daily environmental hassles and greater social support from classmates and parents were significant predictors of less adjustment problems and depression in children with a pediatric rheumatic disease (von Weiss et al. 2002).
Since the initial proposal of Varni’s biobehavioral (biopsychosocial) model, data have been published supporting that the school environment is an additional key intervening variable in the interrelationship of pediatric pain, its precipitants, and resulting functional status (Fig. 11.1) (Kashikar-Zuck et al. 2007; Logan et al. 2008b, 2009). When this Biobehavioral Model of Pediatric Pain was also applied in a predominantly African-American sample of adolescents residing in an urban school system, a significant relationship was observed between anxiety and psychosocial stress and the experience of headache and abdominal pain (White and Farrell 2006). Of note, the Biobehavioral Model of Pediatric Pain is commensurate with the ongoing commitment of the American Academy of Pediatrics (AAP) to the prevention, early detection, and management of behavioral, developmental, and social problems as a priority in present pediatric practice (American Academy of Pediatrics 2001).
The Family Stress Model
In an effort to optimize contemporary pediatric healthcare, attention has also been focused by the AAP on the greater compositional diversity and functional dynamics of the contemporary American family (Schor 2003). The Family Stress Model (Conger et al. 2000) implicitly guided the AAP in generating its current Family Pediatrics: Report of the Task Force on the Family (Schor 2003). The Family Stress Model (Fig. 11.2) is predicated upon the intrinsic interrelationship between socioeconomic status, family processes, and human development. The model acknowledges the strong correlation between lower socioeconomic status (with its attendant economic hardships) and disparities in the cognitive, social, emotional, and physical health and well-being of children, adolescents, and young adults (Conger et al. 2000; Conger and Donnellan 2007). In addressing the resulting detrimental clinical outcomes, the Family Stress Model seeks to identify the biological, psychological, and social resources vs. vulnerabilities that may reduce or intensify the economic stress process (Conger et al. 2000).
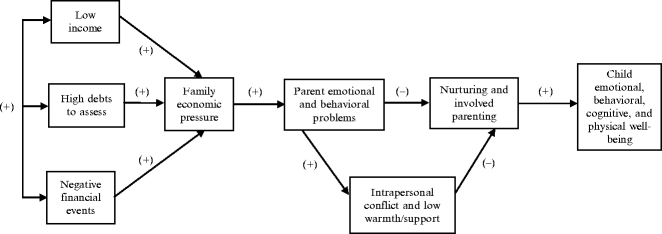

Fig. 11.2
The family stress model applied by the American Academy of Pediatrics Task Force on the Family and germane to the pediatric chronic pain experience. From Conger and Donnellan (2007), p. 180. Reprinted, with permission, from the Annual Review of Psychology, Vol. 58. © 2007 by Annual Reviews www.annualreviews.org
The Family Stress Model is quite germane to understanding the pediatric chronic pain experience, given that family, parental, and situational factors are widely held to play a major integrative role in the natural history of pediatric chronic illness and specifically, the development and perpetuation of chronic pain (Fig. 11.3) (Chambers 2003; Chambers et al. 2002; McGrath and Hillier 2003; Palermo and Chambers 2005). Moreover, contending with a child who is suffering from a chronic medical condition, especially one that is associated with chronic pain and disability, is also a potent parental and family stressor (Eccleston et al. 2004; Jordan 2005). A survey of 533 parents of chronically ill children revealed 45% of them to have health-related quality-of-life impairment, across the domains of sleep, social functioning, daily activities, vitality, positive emotions, and depressive emotions – supporting the need for a family-centered approach in pediatrics (Hatzmann et al. 2008). An effective biopsychosocial approach to pediatric pain is also commensurate with the family-centered pediatric healthcare model that has also been promulgated by the AAP (2003).
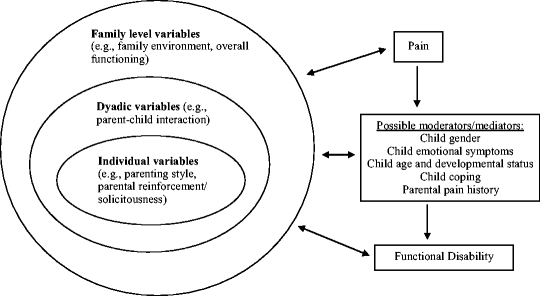

Fig. 11.3
Integrative model of parent and family factors in pediatric chronic pain and its associated disability. From Palermo and Chambers (2005), p. 3. © 2007 by International Association for the Study of Pain. Reprinted with permission from the Elsevier B.V
Current Consensus on the Factors Associated with Pediatric Chronic Pain and Disability
Pediatric chronic pain can have a widespread impact on many aspects of daily living for patients and their families. The actual prevalence of pain and disability in the pediatric populations is not known. The majority of children who do report pain do not tend to show extensive distress and disability. Recent evidence suggests that pediatric patients of all ages, including the extremely premature neonates, are all capable of experiencing pain and subsequent disability as a result of tissue injury, medical illnesses, therapeutic and diagnostic procedures, trauma, and surgery (Davies et al. 2010; Lago et al. 2009; Roth-Isigkeit et al. 2005). Moreover, given the lack of assessment and barriers to treatment of pediatric pain, a minority of youth report pain and disability, including associated depression and maladaptive coping. These issues tend to be more pronounced in younger age groups and in groups with long term, chronic illness. Similar to other studies, sex differences in pain reporting have been observed, with girls tending to report pain in greater numbers than boys (Eccleston and Clinch 2007; see also, Keogh 2011).
A recent Delphi poll of professionals with a specific interest in chronic pain in children and adolescents was undertaken to reach a consensus as to the factors associated with pediatric chronic pain and disability (Miro et al. 2007). Factors deemed most important by these stakeholders in the development of chronic pain included: (1) child’s psychological characteristics: the child’s tendency to somaticize, depressed personality, and anxious personality; (2) parent’s psychological characteristics: parental emotional instability; (3) characteristics of the pain experience: suffering from constant pain, and a family history of chronic pain; (4) characteristics of pain management: an excessive use of healthcare services for the child pain complaints, an inappropriate consumption of medicines to relieve the pain, doctor searching for the pain problem without finding anything wrong, and a low compliance with the healthcare professionals’ recommendations; (5) psychological factors related to the child’s pain experience: catastrophic thinking of the child and parents about the child’s pain, child’s negative expectations about the course of her or his pain problems, and the presence of positive reinforcements in response to the child’s pain behaviors; and (6) a stressful environment (e.g., due to family difficulties, problems at school) (Miro et al. 2007). By the same token, the consensus factors considered by these stakeholders to be most important in the development of a disability include: (1) child’s psychological characteristics: the tendency to somaticize, and a depressed personality; (2) parents’ psychological characteristics: high anxiety; (3) psychological factors related to the child’s pain experience: pain-related fear avoidance (child’s and parents’ catastrophic thinking about the child’s pain, and child’s avoidance behaviors due to the fear of experiencing more pain), child’s beliefs about her or his being disabled by the pain, and pain modeling factors (parents’ avoidance behaviors due to the fear of experiencing more pain, parents’ belief that pain incapacitates, and parents’ own disability because of pain); and (4) parents’ ability to deal with her or his child’s pain (Miro et al. 2007).
Health-Related Quality of Life and Pediatric Chronic Pain
Pediatric chronic pain is a complex and individual and social experience (Graumlich et al. 2001; Malaty et al. 2005; Schechter et al. 2003). It is often difficult for patients and their parents to fully describe the impact of the experience and meaning of chronic pain using only self-reported or observational unidimensional pain intensity scales (Gaffney et al. 2003; Stevens 1994). As noted above, chronic pain has a substantial adverse impact on children and adolescents, resulting in significantly worse physical functioning, psychological functioning, social functioning, in addition to lower satisfaction with life and poorer self-perceived health status (Merlijn et al. 2006; Palermo 2000).
In contrast to more conventional physiologic, laboratory, and radiological assessments of disease, the measurement of health-related quality of life (HRQoL) can provide equally important insight into these adverse effects of pediatric chronic disease, especially those associated with pain and disability (Eiser and Morse 2001b, c; Guyatt et al. 1993; Vetter 2007). Sequentially assessing HRQoL can also provide clinicians, their patients, and their parents with additional information about the longitudinal effectiveness of a chronic pain treatment regimen and assist in point-of-service clinical decision-making (Drotar 2004a; Varni et al. 2005; Vetter 2007).
Health-related quality of life has become a central issue in the effective assessment, treatment, and management of pediatric chronic diseases, and it has been posited that the measurement of HRQoL should be routine in pediatric outcomes research and clinical practice (Eiser 2004; Palermo et al. 2008; Parsons and Mayer 2004). The Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) has recently recommended that investigators conducting pediatric clinical trials in chronic and recurrent pain should consider assessing outcomes in pain intensity; physical functioning; emotional functioning; role functioning; symptoms and adverse events; global judgment of satisfaction with treatment; sleep; and economic factors (McGrath et al. 2008).
Nevertheless, the measurement of HRQoL in pediatric research and clinical practice has remained limited as compared to its measurement in the adult population (Clarke and Eiser 2004; Varni et al. 2005). Conceptual and methodological issues have hindered the routine assessment of HRQoL in chronically ill children and adolescents, including those suffering from chronic pain (De Civita et al. 2005; Drotar 2004a; Matza et al. 2004). One fundamental issue is the central yet complex role of child development and the associated dynamic social and psychological contexts in which a child or adolescent perceives health vs. disease (Forrest et al. 2003). Specifically, parents, siblings, and peers, as well as the classroom setting and the community, can all play an important role in the self-perceived HRQoL of a child or adolescent (Matza et al. 2004; Palermo 2000).
These methodological challenges can be overcome, however, if sufficient consideration is given to the choice of an age-appropriate pediatric HRQoL instrument (Drotar 2004b; Eiser and Morse 2001b; Landgraf and Abetz 1996; Vetter 2007). The use of an adult generic HRQoL measure (e.g., 36-Item Short-Form Health Survey, SF-36) should be avoided in chronic diseases of childhood due to its likely failure to tap important pediatric health domains and its response burden (Eiser and Morse 2001c). A parental proxy assessment of a younger child’s HRQoL is a viable alternative. However, children 8–11 years of age appear to report significantly lower HRQoL than their parents (Theunissen et al. 1998). Therefore, whenever possible, in addition to those of a parental proxy, the pediatric patient’s own health perceptions should also be elicited (Eiser and Morse 2001a). If the health survey instrument is appropriately structured, a child as young as 4 years of age can provide meaningful, even if only concrete insight into their self-perceived health status, including their experience of pain. More subjective or abstract health domains can be self-reported by individuals 8 years of age and older (Eiser et al. 2000; Matza et al. 2004; Vetter 2007).
Previous pediatric studies have also observed an imperfect concordance or cross-informant variance in patient self-reported health-related quality vs. parent proxy-reported health-related quality (Knight et al. 2003). This discordance has been observed not only in healthy subjects (Cremeens et al. 2006), and in a community adolescent sample (Waters et al. 2003), but also in patients specifically with functional abdominal pain (Youssef et al. 2006), cancer (Varni et al. 2002; see also Ransom et al. 2011), and sickle cell pain (Panepinto et al. 2005). Nevertheless, despite the attendant methodological challenges, the assessment of HRQoL represents a vital element of pediatric outcomes research (Palermo et al. 2008).
The Biopsychosocial Characteristics of Pediatric Chronic Pain Patients: Additional Barriers to Its Effective Management
A consistent clinical profile has been observed over the last decade among patients referred to four tertiary-care multidisciplinary, pediatric pain medicine clinics (Eccleston et al. 2004; Kashikar-Zuck et al. 2001; Logan et al. 2009; Vetter 2008). All four cohorts were likely fraught with selection bias due to referral patterns, and cancer-related pain was conspicuously absent in these published study samples. Nevertheless, collectively, they still offer important insight into the biopsychosocial characteristics of pediatric chronic pain.
In a group of 73 patients presenting to a pediatric pain medicine clinic, most were adolescents, Caucasian (90%), female (78%), and 45% had pain of greater than 24 months duration. While many reported more than one pain location, back pain (18%), abdominal pain (16%), limb pain (15%), and myofascial pain (15%) were the most common primary types of pain. Most patients reported at least mild to moderate levels of depression, and approximately 15% of the patients reported severe levels of depressive symptoms. Based upon the Functional Disability Inventory (FDI), approximately 75% of study subjects self-reported moderate to high levels of functional disability (FDI score > 10), including disruption in psychosocial functioning (Kashikar-Zuck et al. 2001).
In a second group of 80 pediatric patients with chronic pain who were referred for assessment at a specialized tertiary care chronic pain management service, the majority (71%) were girls, with a mean age of 14.5 years, whose pain had persisted for an average of 3.9 years. Using a chronic pain syndrome diagnostic system (rather than more conventional ICD-9 coding) (Malleson and Clinch 2003), four conditions were most common: complex regional pain syndrome (CRPS) type 1 (26%), juvenile widespread idiopathic musculoskeletal pain (24%), recurrent abdominal pain (12%), and low back pain (12%). Based upon their responses on a battery of previously validated questionnaires, these adolescents frequently reported clinically significant levels of disability (85%), depression (31%), and anxiety (50%), and their parents reported similarly frequent high levels of depression (40%), anxiety (60%), and parenting stress (66%). Multiple regression analyses revealed that the strongest predictors of this adolescent emotional distress were the extent to which the adolescents catastrophize and seek social support to cope with the pain (Eccleston et al. 2004).
A third such cohort of 100 pediatric pain patients were predominantly adolescent (median age of 14 years) females (68%), whose median duration of chronic pain was 16 months. Low back pain (14%), myofascial pain or fibromyalgia (14%), abdominal pain (12%), and headache (8%) were most predominant. A substantial proportion of these patients exhibited evidence on psychological testing of clinically significant anxiety (63%) and/or depression (84%) at the time of their initial evaluation. The mean initial patient self-reported and parent proxy-reported health-related quality of life scores (PedsQL Total Score) were also significantly lower than the PedsQL Total Score values previously observed in pediatric rheumatology, pediatric migraine, and pediatric cancer patients being treated in single subspecialty clinics (Vetter 2008). This is not surprising given that such anesthesiology-based, multidisciplinary pediatric chronic pain medicine programs appear to often function as “the court of last resort” for particularly clinically enigmatic and challenging patients and families. This phenomenon was reflected in the rather low self-reported parent healthcare satisfaction with previous, ostensibly inadequately effective chronic pain-related treatment (Vetter 2008). Such intense chronic pain-related symptoms and disability mandate a robust multidisciplinary care team approach, which focuses on both the biological, psychological, and social pathology and needs of the pediatric patient and the family.
A fourth published sample of 217 patients, who presented for initial evaluation of chronic or recurrent functional (i.e., nonmalignant) pain symptoms at a similar tertiary pediatric chronic pain outpatient clinic within a large urban children’s hospital, were primarily female (80%) and Caucasian (93%), with a 22-month mean duration of pain. Based upon responses on the Child Depression Inventory (CDI), only moderate levels of depressive symptoms were observed overall, with just 20% of the sample falling in the at-risk or clinically significant range of depressive symptoms. Nevertheless depressive symptoms strongly correlated with a variety of school functioning indicators, supporting the study hypothesis that depressive symptoms play a key role in influencing the degree of school impairment in adolescents with chronic pain (Logan et al. 2009).
It bears worth noting that data indicate that such adolescents presenting for evaluation and treatment of chronic pain may minimize self-reported psychological distress due to a social desirability response bias (Logan et al. 2008a). Furthermore, it has been previously observed that adolescent pain patients may infrequently exhibit diagnosable levels of depression, but that depressive symptoms, even at sub-clinical levels, may be clinically significant because they indicate poorer emotional coping resources (Eccleston et al. 2004). Given the findings in this and previous studies that show depressive symptoms are associated with functional disabilities in the presence of chronic pain, even sub-clinical levels of depressive symptoms deserve greater attention and represent a potentially important targets for treatments aimed at functional restoration and rehabilitation rather than simply a reduction in pain intensity (Logan et al. 2009). Thus, formal psychological assessment for depression, as well as anxiety, using a conventional scoring tool and/or a patient interview (Holmbeck et al. 2008; Kazdin 2005; Rudolph and Lambet 2007; Southam-Gerow and Chorpita 2007), should be part and parcel of the initial and the ongoing evaluation of the pediatric chronic pain patient.
The Effects of Race and Ethnicity on Pediatric Chronic Pain
Race is a social category based on similar ancestry and physical characteristics, while ethnicity is based also on shared behavior and culture (Edwards et al. 2001). It is widely accepted that racial and ethnic disparities in pediatric health and healthcare are extensive, pervasive, and persistent (Flores 2010; see also Green 2011). Although racial and ethnic healthcare disparities existing among adults with chronic pain are well documented (Anderson et al. 2009), published epidemiological data on pediatric chronic pain have been drawn almost exclusively from nonminority, middle-class populations outside the United States (Vetter 2011). Furthermore, little attention has been focused on race, ethnicity, socioeconomics, and the social environment as likely causal factors in the onset, persistence, and impact of pediatric chronic pain (Fortier et al. 2009; Schwartz et al. 2007).
The Qualitative Nature of Pediatric Chronic Pain and the Patient’s Personal Narrative
Because of the innately subjective and hence qualitative nature of pediatric chronic pain, research attention has also recently focused on collecting complementary data from semi-structured, qualitative patient interviews and patients’ personal narratives to gain a better understanding of the impact of pain-associated functioning limitations on children’s lives and the strategies they develop to try to continue functioning.
A mixed-methods approach was applied in a group of 45 children (71% female, 64% Caucasian, mean age of 14.7 years), who presented a tertiary university-based pediatric pain clinic suffering predominantly from headache, myofascial pain, and/or functional neurovisceral pain disorder (Meldrum et al. 2008). Analysis of the data derived from standardized questionnaires and semi-structured interviews identified three distinct functioning distinct patterns or groups of functional limitation and coping strategies, which were designated as Adaptive (29%), Passive (29%), and Stressed (58%). These three groups did not differ significantly in demographics or clinical pain characteristics. Distinct differences emerged. Adaptive children continued to participate in many activities, often because of more effective use of distraction and of other independently developed strategies to continue functioning. The adaptive children were also more likely to realize that focusing on pain would heighten their perception of pain. Passive children had already given up most activities, tended to use passive distraction when in pain, and were more likely to feel isolated and different from peers. Stressed children described themselves as continuing to function, but were highly focused on their pain and the difficulties of daily living with it. Parent ratings of their child’s global health on the Child Health Questionnaire (CHQ PF50) also differed significantly across the groups; with the Adaptive group exhibiting higher CHQ PF50 scores and thus better perceived global health than the Passive group. Child self-report total scores Social Anxiety Scale for Children (SASC) also differed significantly across the groups, with the Stressed group reporting higher SASC scores and higher overall social anxiety than the Passive group (Meldrum et al. 2008).
While likely not practical in routine clinical practice, the use of patient narrative in clinical therapy and research (i.e., “narrative medicine”) can provide valuable insight into the human pain experience (Carr et al. 2005; see also Morris 2011). Such a narrative method has recently been used to better understand the impact of chronic or recurrent pain on children within the context of their own lives and experiences, specifically, in 53 children (68% female; 68% Caucasian, mean age 14.2 years), who presented a tertiary university-based pediatric pain clinic suffering predominantly from headaches, functional neurovisceral pain disorder, and/or myofascial pain (Meldrum et al. 2009). Based upon a 30–90 min, semi-structured life history interview at home prior to their first pain clinic visit and a follow-up interview at 6–12 months post-intake, five common themes of the children’s chronic pain experience emerged (Table 11.1).
Theme: frequent patient expression |
|---|
The choice to hide pain from parents and friends: “I don’t want to be a sob story” |
A sense of isolation and difference from peers and classmates: “I’m different and everybody stares and stuff” |
Pain as an obstacle to personal activities and goals: “It keeps me from doing a lot of things I like” |
Fears about how pain will affect the future: “I’m scared I might always have this problem” |
Perceived lack of physician understanding: “I think about why it’s called the medical practice” |
Using narrative methodology similar to the seminal adult work on narrative by Frank (1995), six major, richly detailed descriptive pediatric chronic pain narratives, with unique characters and plotlines were in turn identified (Table 11.2). These narratives are consonant with previously published studies (Aasland et al. 1997; Hunfeld et al. 2002a; Mulvaney et al. 2006). These findings validate the conventional clinical belief that patient isolation, changed self-perception, activity limitations, concerns about barriers to future goals, and lack of medical validation are vital to children’s perception of the biopsychosocial impact of chronic pain on their lives (Meldrum et al. 2009).
The character | The plotline |
|---|---|
The constant patient | The medical narrative |
The invalid | Defeated by the pain |
The weary soldier | Fighting constant stress |
The stoic | “Pushing through” the pain |
The positive thinker | One step at a time |
The decision-maker | The major rewrite |
The Pragmatic Goal of Pediatric Chronic Pain Management
The more pragmatic goal of pediatric chronic pain management is the timely return of a patient to as normal and functional a life as possible – with an associated improvement in health-related quality of life – while at the same time reducing the attendant stress on the parents and other family members (Bennett et al. 2000; Hunfeld et al. 2001, 2002b; Palermo 2000; Varni et al. 1996a). Indeed, at the time of their initial evaluation, patients and their parents naturally seek the complete elimination of the child or adolescent’s presenting pain. Yet, this clinical outcome is unfortunately often unobtainable, with upwards of 48 and 30% of pediatric patients reporting persistent pain at 1- and 2-year follow-up, respectively (Perquin et al. 2003). Moreover, youngsters with chronic pain often go on to experience chronic pain as young adults. In a longitudinal study of a cohort of children 8, 11, and 14 years of age, suffering from chronic headache or chronic back pain, 59% of the females and 39% of the males reported similar pain at 21, 24, and 27 years of age (Brattberg 2004).
A Synopsis of Biomedical and Psychosocial Modalities in Pediatric Management
The effective assessment, treatment, and management of the myriad of chronic pain conditions that afflict children and adolescents are a very complex clinical endeavor (Zeltzer et al. 1997b, c). The management of pediatric chronic pain and its attendant patient disability and family dysfunction optimally follows a multidimensional and multidisciplinary approach (Schechter et al. 2003), which is specifically tailored to the identified medical, psychological, and social needs of each patient and family (Bennett et al. 2000; Bursch et al. 1998). Any such comprehensive pediatric pain management algorithm calls for an interdisciplinary approach that includes rehabilitation, as well as psychosocial therapies, and when indicated, analgesic and adjuvant medications, an increasing use of complementary and alternative methods, and interventional techniques (Knotkova and Pappagallo 2007).
While an extensive review of available modalities is beyond the scope of this chapter, a brief integrative synopsis is provided. For more in-depth coverage, the reader is referred to available general therapeutic reviews (Chambliss et al. 2002) and comprehensive textbooks (Finley 2006; McClain and Suresh 2010; Walco and Goldschneider 2008), as well as focused reviews on pediatric chronic headache (Hershey et al. 2006, 2007; Lewis 2009; Winner 2008), musculoskeletal pain (Clinch and Eccleston 2009; Connelly and Schanberg 2006; Malleson and Clinch 2003), fibromyalgia (Buskila 2009), neuropathic pain (Walco et al. 2010), and abdominal pain (American Academy of Pediatrics 2005; Berger et al. 2007).
Biomedical Modalities: Analgesic Medications
Due to an unfavorable risk–benefit ratio (i.e., an inadequate economic return to justify the cost of required additional clinical trials and potential medical liability), pharmaceutical companies in the United States and the European Union have infrequently sought regulatory approval of their novel analgesics or adjuncts for use in the pediatric chronic pain population (Breitkreutz 2008; Li et al. 2007; Manolis and Pons 2009). This phenomenon has resulted in common pediatric off-label medication use (Benjamin et al. 2006; Milne and Bruss 2008; Roberts et al. 2003). Despite greater recent studies and labeling changes of pediatric medications (e.g., the FDA Pediatric Exclusivity Program) (Benjamin et al. 2009), the majority of pediatric outpatient visits involve off-label prescribing across all medication categories, with off-label prescribing more frequent for younger children and those receiving care from specialist pediatricians (Bazzano et al. 2009).
Even in adults, many current analgesic medications (including notably gabapentin and amitriptyline) are used off-label, with clinicians relying upon at best small-scale investigator-initiated trials in deciding whether to prescribe a medication (Chen et al. 2009; Radley et al. 2006). Rationale polypharmacy, based upon mechanistic stratification (Beydoun and Backonja 2003) and a stepped care approach (Kroenke et al. 2009), is typically applied in adults with chronic pain, with the goal of maximizing clinical benefit and minimizing side effects (Varrassi et al. 2010). A similar patient-individualized, trial-and-error strategy is also typically applied in children and adolescents with chronic pain. More preclinical, clinical, and translational (population) studies are needed to improve the efficacy of combination drug therapy that has become an integral part of a comprehensive approach to the management of chronic pain in adult and pediatric pain populations (Mao et al. 2011).
Tramadol
The routine use of tramadol warrants consideration in pediatric chronic pain (Bozkurt 2005). When given postoperatively as a single 1 or 2 mg/kg oral dose in children between the ages of 7 and 16 years, tramadol has demonstrated a narcotic dose-sparing effect, with a minimal adverse side effect profile (Finkel et al. 2002). An open-label, multicenter trial of the safety profile and analgesic efficacy of tramadol in 7–16-year olds for the treatment of painful conditions lasting up to 30 days found that tramadol 1–2 mg/kg orally every 4–6 h (maximal dose of 8 mg/kg/day, not to exceed 400 mg/day) to be a safe and effective analgesic in this patient population (Rose et al. 2003).
Tramadol must be converted by the cytochrome P450 2D6 (CYP2D6) isoenzyme to its active metabolite, O-demethyl tramadol (M1), to provide analgesia (Grond and Sablotzki 2004). Of note, CYP2D6 metabolizes approximately 25% of current drugs, including some commonly prescribed antidepressants (e.g., fluoxetine, paroxetine, all of the tricyclic antidepressants [TCAs]) and opioids (e.g., codeine, hydrocodone, and methadone) (Caraco 2004; Weinshilboum 2003). CYP2D6 activity ranges considerably within a general population and among different ethnic groups, with ultrarapid metabolizers, extensive (normal) metabolizers, intermediate metabolizers, and poor metabolizers (Gardiner and Begg 2006; Zhou 2009a, b). Because of this genetic polymorphism, large inter-individual variation in the enzyme activity exists, with 1.5–10% of Caucasians, 1.9–7.3% of African-Americans, 1.8–19% of Africans, 0–2–4.8% of Asians, and 2.2–6.6% of Hispanics lacking sufficient CYP2D6 enzymatic activity and thus being poor metabolizers of its substrates (Bernard et al. 2006; Zhou et al. 2009). This inability to metabolize via CYP2D6 is one factor that also contributes to inadequate pain management across ethnically diverse patient populations.
The effect of CYP2D6 activity in drug metabolism is further complicated by the activity of a drug itself on CYP2D6, as CYP2D6 inhibitors may make extensive (normal) metabolizers act like intermediate metabolizers or poor metabolizers. Thus, the co-administration of tramadol and a number of medications, including methadone, any of the TCAs, duloxetine, and many of the selective serotonin reuptake inhibitors (SSRIs), can result in seizures, due to inhibition of CYP2D6 and the accumulation of tramadol, or serotonin syndrome (Sansone and Sansone 2009; Tashakori and Afshari 2010), even at their recommended doses.
Transdermal Fentanyl
A number of myths and obstacles, including fears of addiction or diversion, continue to hamper the prescription of opioids for pediatric cancer pain (Friedrichsdorf and Kang 2007) and for chronic nonmalignant (noncancer) pain that is refractory to other analgesics (Table 11.3) (McGrath and Ruskin 2007). Whereas most adults actively seek pain relief, children may cope with pain by withdrawing, rather than crying or asking for medication. Once routine, pediatric intramuscular injections are fortunately now less common, for their use prompts many children to not complain of pain for fear that the nurse’s answer to their pain would be a dreaded analgesic injection (“a shot”). Nevertheless, children often believe they deserve their pain as punishment for something they did wrong (Rice 1996). Children and adolescents have widely variable individual pain perceptions of similar conditions, often resulting in widely different analgesic needs. While these differences are likely based on a combination of past experiences, culture, and genetics (Brislin and Rose 2005), additional insight is needed as to how best to assess and to address these predilections (Kraemer and Rose 2009).
Table 11.3
Myths and obstacles to prescribing opioids for pediatric chronic pain (Friedrichsdorf and Kang 2007)
Parents |
•Fear of giving up |
•Misconceptions of opioids as “too strong for children” |
•Fear of side effects |
•Worry their child will become “addicted” to pain medications |
•Cultural or religious beliefs |
Healthcare providers |
•Lack of sufficient education regarding managing pain |
•Misconceptions about frequency and severity of side effects, such as respiratory depression |
•Worries that opioids will shorten life expectancy |
•Concerns that escalating opioid doses will increase the likelihood of tolerance, and thus make pain control more difficult as the disease progresses |
While transdermal fentanyl has been used for pediatric cancer pain and palliative care (Collins et al. 1999; Finkel et al. 2005; Zernikow et al. 2007), this unique drug delivery system also has a place in the management of noncancer pain. A recently introduced transdermal fentanyl patch with a lower drug release rate of 12.5 μg/h has made it more applicable in the pediatric population (Zernikow et al. 2009). However, while observational clinical and pharmacokinetic studies have been published, at this time, no pediatric randomized or controlled cohort studies have been reported (Zernikow et al. 2007).
Tricyclic Antidepressants
Despite a paucity of published randomized controlled trials, the TCAs are widely prescribed for a variety of pediatric chronic pain conditions. The TCAs are frequently used as the first-line drug of choice for pediatric chronic pain. Pain relief is often seen at lower doses (75 mg in adults) than required for the treatment of depression or generalized anxiety (Bryson and Wilde 1996). However, the TCAs have a relatively narrow therapeutic margin. An electrocardiogram (ECG) should be obtained in all patients prior to initiating a TCA to rule out Wolf-Parkinson-White (WPW) and a prolonged QT syndrome, heart rhythm disorders that can potentially cause a fast, chaotic heartbeat and are contraindications to the use of a TCA. A repeat ECG is indicated at a TCA dose of greater than 1 mg/kg/day. The TCAs are metabolized primarily by the CYP2D6 isoenzyme, with amitriptyline metabolized to nortriptyline, and imipramine metabolized to desipramine. The secondary amine TCAs (nortriptyline and imipramine) are less sedating than the tertiary amine TCAs (amitriptyline and imipramine), which often determine clinical preference. The usual starting dose of a TCA is 10 mg/day (0.15 mg/kg/day), with an analgesic target dose of 1 mg/kg/day. Nortriptyline is commercially available as a liquid solution (10 mg/5 mL), facilitating its weight-based dosing in children. The TCAs are equipotent, which facilitates conversion from one agent to another (e.g., nortriptyline in lieu of amitriptyline for less sedation).
As noted above, genetic polymorphism of the CYP2D6 enzyme exists, with some patients being poor (slow) metabolizers of the TCAs (Bernard et al. 2006; Zhou et al. 2009). Therefore, a plasma TCA level should be obtained when a dose of 0.5 mg/kg/day is reached, and if the plasma TCA level is within the therapeutic reference range (100–250 ng/mL for amitriptyline and nortriptyline or 50–150 ng/mL for nortriptyline alone) (Lexi-Comp 2010; Wilson 2003), the daily dose should not be further increased. The parent molecule and its metabolite are additive in therapeutic effect but also toxicity. While the TCAs are often discounted as being excessively side effect prone, the newer serontonin-norepinephrine reuptake inhibitors (SNRIs) like venlafaxine (Effexor®), duloxetine (Cymbalta®), and milnacipran (Savella®) have a similar dose-dependent anticholinergic (muscarinic receptor antagonist) side effect profile (Kong and Irwin 2009; Kroenke et al. 2009; Verdu et al. 2008).
Antiepileptic Drugs
Based upon available adult studies (Hauser et al. 2009; Wiffen et al. 2005), gabapentin (Neurontin®) is a potential and promising pediatric analgesic adjuvant. Gabapentin is FDA-approved as an adjunctive therapy in the treatment of partial seizures in pediatric patients 3 years of age and older. Gabapentin is commercially available as a 100 mg capsule and a liquid solution (250 mg/5 mL), facilitating its weight-based dosing in children. In adults an initial gabapentin dose of 300 mg every 8 h, titrated as indicated in 100–300 mg increments up to 600–1,200 mg every 8 h, appears optimal. Extrapolating from adult studies and applying an ideal adult body weight of 60–70 kg, an initial pediatric oral dose of 5 mg/kg every 8 h, titrated slowly up to 20 mg/kg every 8 h, appears reasonable. This gabapentin dose is commensurate with that for the treatment of pediatric epilepsy and has supportive pediatric pharmacokinetic data, with a therapeutic plasma level of 5–15 μg/mL (Haig et al. 2001; Holmes 1997; Hwang and Kim 2008). Of note, plasma gabapentin levels can be readily obtained, whereas plasma pregabalin (Lyrica®) levels are not commercially available. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug and is not appreciably metabolized in humans. As in adults, reduced gabapentin doses are mandated in patients 12 years of age or older with renal dysfunction. Gabapentin should not be used in children less than 12 years old with compromised renal function.
While gabapentin has the greatest clinical track record of use for pediatric chronic pain conditions (albeit completely off-label), published data also support the use of at least two other antiepileptic drugs. Topiramate (Topamax®) has been shown to be more effective than placebo for the prophylaxis of pediatric migraine (Cruz et al. 2009; Lakshmi et al. 2007; Lewis et al. 2009; Winner et al. 2005). While its reported use for pediatric chronic pain is very limited (Lalwani et al. 2005), oxcarbazepine (Trileptal®) has gained acceptance for pediatric epilepsy (Chung and Eiland 2008; Franzoni et al. 2009; Kothare et al. 2006; Pina-Garza et al. 2005). While hyponatremia is less commonly observed in children and adolescents than in adults taking oxcarbazepine, nevertheless, serum sodium levels should be monitored with its use.
Psychosocial Modalities
Individual and/or family therapy, especially using a cognitive-behavioral or coping-skills model, has been shown to be effective in treating a broad-spectrum of common pediatric chronic pain conditions (Eccleston et al. 2003, 2009), including, specifically, headache (Andrasik and Schwartz 2006; Holroyd and Drew 2006; Powers and Andrasik 2005; Rosen 2008), fibromyalgia (Degotardi et al. 2006; Kashikar-Zuck et al. 2005), recurrent abdominal pain (Duarte et al. 2006; Huertas-Ceballos et al. 2008; Levy et al. 2010; Robins et al. 2005), and CRPS (Lee et al. 2002).
Nevertheless, it is vital to recognize that these psychological therapies may not be readily available due to geographic location, or an affordable option for many families of children and adolescents with chronic pain, and that many parents and children may be quite resistant to any such treatment recommendation that seems to imply that the pain is somehow “not real” or “all in my head” (Meldrum et al. 2009). One potential way to circumvent these financial constraints and geographic proximity is to utilize an Internet-based delivery method (Long and Palermo 2009). This method has been shown to have some efficacy in pediatric cancer patients and in pediatric pain populations (see also Quintana and Jane, this volume). When compared to a wait-list control regimen, Internet delivery of family cognitive behavioral therapy was shown to be more efficacious in reducing pain intensity and improving functional disability in a group of 48 older children and adolescents, aged 11–17 years, with chronic headache, abdominal, or musculoskeletal pain and associated functional disability (Palermo et al. 2009). Similar greater benefit was observed in 65 adolescents headache patients who received two different self-help training programs (multimodal cognitive-behavioral training and applied relaxation) presented via the Internet as compared to a conventional educational intervention (Trautmann and Kroner-Herwig 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





