Key Clinical Questions
What are the diagnostic criteria for idiopathic Parkinson disease?
What is the differential diagnosis of a patient with parkinsonian symptoms?
What is the pathophysiology of Parkinson disease?
What treatments are available? How do they relate to the underlying pathophysiology?
What side effects and long-term effects may occur in a patient with Parkinson disease who is on medication?
What complications may occur when a patient with Parkinson disease is admitted to the hospital?
Introduction and Epidemiology
Parkinson disease (PD) develops in 12 to 20/100,000 people yearly, with a prevalence as high as 1% in those older than 65 years. With appropriate treatment, there appears to be no significant effect on life expectancy. However, quality of life is markedly reduced due to a variety of factors, some of which have only recently been recognized, such as autonomic system dysfunction.
Estimates of the economic burden of PD in various countries range from $4,000 to $20,000 per patient yearly, including roughly 33% to 40% in indirect costs, such as lost productivity. On average, PD-affected patients can no longer work full time by 3.4 years after diagnosis, and most file for disability by 5 years. In addition, PD is a risk factor for nursing home placement.
Pathophysiology
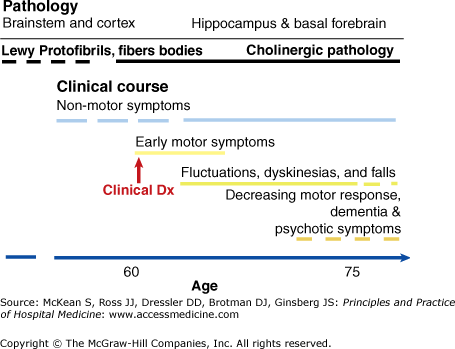
PD is associated with the loss of dopaminergic neurons located in the midbrain, in the substantia nigra pars compacta (SNc). Patients who are still early in their clinical course have lost ˜80% of the SNc neurons, implying that the degenerative process begins long before motor symptoms appear, and that the basal ganglia are able to compensate until there is a critical loss of dopaminergic neurons. How the loss of dopamine disrupts normal functioning of the basal ganglia is not fully understood, but it appears to result in pathologic synchronization of neuronal firing throughout the region that interferes with both voluntary and involuntary movements. PD has many nonmotor symptoms as well, suggestive of a more widespread neurodegenerative process involving neurotransmitters other than dopamine. There is increasing evidence for more extensive involvement of the brainstem and cortex, perhaps explaining the effects of PD on cognition, behavior, and sleep.
In most cases, the cause of PD is unknown. Mutations in single genes such as parkin, DJ-1, and LRRK2 are responsible for only a minority of cases of PD but are more prominent in patients who develop symptoms before the age of 50. The relationship of these mutations to neuronal death is not known, but it may involve several mechanisms, including protein misfolding, defective protein degradation by proteosomes, and increased oxidative stress. Recently, an association of PD with relatively common mutations in the gene for the lysosomal enzyme glucocerebrosidase has been described.
The pathological hallmark of PD is the presence of Lewy bodies in the neurons of the SNc. Lewy bodies may be more widely distributed and are found in the cortex in the related disorder known as dementia with Lewy bodies. Lewy bodies are composed of intracellular clumps of proteins, including alpha-synuclein and ubiquitin. Alpha-synuclein is thought to be a phospholipid-binding protein that helps vesicles dock with the plasma membrane. It is not known whether Lewy bodies are toxic to the neurons in which they develop; more likely, they are a mechanism for keeping damaged proteins out of the way of normal cellular functioning.
Differential Diagnosis of Parkinsonism: Parkinson-Plus Syndromes
The term parkinsonism refers to the presence of signs of idiopathic PD, such as slowness of movement (bradykinesia), absence of movement (akinesia), increased tone (rigidity), resting tremor, and impaired postural reflexes. Some or all of these features may be seen in other neurodegenerative disorders, known as the Parkinson-plus syndromes, or atypical parkinsonism. The term secondary parkinsonism is used when another etiology has been identified, such as medication or cerebrovascular disease.
A 55-year-old man presents with gradual onset of stiffness and dizziness. When he gets up from bed or from the table, he needs to stand still for a minute or two, or he would faint. He has difficulty walking and reports that this is because he feels as if he “is on a boat.” He reports loss of sexual function for 3 years with no clear cause. Examination is notable for orthostatic hypotension, limb stiffness, and ataxic gait. |
Patients with multiple system atrophy (MSA) may have varying combinations of parkinsonism, cerebellar disease, and autonomic dysfunction that may resemble idiopathic PD, especially in the early stages. MSA-parkinsonism (MSA-P) involves striatonigral degeneration (rather than nigrostriatal, as seen in PD). Although MSA-P predominantly affects the neurons postsynaptic to the dopaminergic neurons, patients seem to be at least partly responsive to levodopa therapy. MSA-cerebellar (MSA-C) mostly affects the olivocerebellar connections, with disproportionate involvement of the middle cerebellar peduncles. These tracts originate in the pons and travel to the cerebellar cortex. MSA-autonomic (also called Shy–Drager syndrome) is characterized by degeneration of the locus coeruleus, the dorsal motor nucleus of the vagus nerve, and the catecholamine-producing neurons of the ventrolateral medulla.
Pathologically, alpha-synuclein inclusions are found in glial cells, rather than neurons as in PD. The locations of the inclusions determine the clinical spectrum of disease and can include the SNc, locus coeruleus, putamen, inferior olives, pontine nuclei, cerebellar Purkinje cells, and intermediolateral columns in the spinal cord.
MSA is usually not inherited, and the cause is unknown. There is no disease-modifying treatment, and treatment is symptomatic. Parkinsonian patients may have some response to levodopa, which should be increased to the maximum tolerated dose. Autonomic dysfunction is managed by giving midodrine, a vasoconstrictor, and mineralocorticoids such as fludrocortisone to increase blood pressure, with antispasmodics or catheters for urinary incontinence. Physical and speech therapy are often useful. As with all parkinsonian disorders, swallowing is often affected and should be evaluated to reduce the chances of aspiration, a major cause of morbidity and mortality.
A 68-year-old woman presents with difficulty walking and abrupt falling. She has been falling spontaneously with increasing frequency, especially when walking down stairs. She also has been having trouble swallowing. On examination, her neck is stiff and slightly extended, with milder stiffness of the wrists. Her eye movements seem somewhat slowed, with decreased ability to look down, and her overall demeanor is muted. |
In its early stages, progressive supranuclear palsy (PSP) may be indistinguishable from PD, but axial symptoms such as gait impairment and balance problems are usually prominent and severe earlier in the course. Prominent features of PSP include supranuclear palsy with early impairment of vertical gaze, mild dementia with frontal features, dysarthria, and dysphagia. PSP has been linked to genetic variants in tau, a microtubule-associated protein. Pathologically, the brain shows inclusion bodies made up of tau protein, along with tufted astrocytes and globose tangles. PSP (like most atypical parkinsonian syndromes) shows a poor response to levodopa, but this should be tried as one of the few available pharmacotherapeutic options. Early involvement of a speech therapist is important.
|
In dementia with Lewy bodies (DLB), patients present with features identical to idiopathic PD but have early dementia and behavioral abnormalities out of proportion to the accompanying motor symptoms. Visual hallucinations, which are usually benign and nondistressing, are a typical early feature. Pathologically, DLB resembles PD, in that the cytoplasm of affected cells has similar Lewy body inclusions, but these are more widely distributed, and the affected area includes the cerebral cortex, in addition to the SNc. Treatment is similar to that for Alzheimer disease (acetylcholinesterase inhibitors) and to the other parkinsonian syndromes.
|
Corticobasal degeneration (CBD) is a tauopathy that manifests with parkinsonian symptoms and dementia. There can be cortical symptoms, such as apraxia, aphasia, higher order sensory loss, or alien hand syndrome, where one limb may writhe or perform complex involuntary movements, such as picking at the hair or clothes. Patients may have asymmetric parkinsonism or limb dystonia. Imaging shows asymmetric atrophy of the frontoparietal cortex; pathologically, there are ballooned, achromatic neurons. Corticobasal syndromes may feature in other conditions, such as Alzheimer disease and PSP. The neurologic symptoms are related to the distribution of the tau-immunoreactive inclusion bodies in the cortex and basal ganglia.
Other Causes of Parkinsonism
Microvascular changes and small infarcts from atherosclerosis may disrupt the fibers of the basal ganglia, leading to parkinsonian symptoms without degeneration of dopaminergic neurons. This is usually seen in patients with typical vascular risk factors, such as hypertension, diabetes, hyperlipidemia, smoking, and older age. Patients with vascular parkinsonism generally have early gait problems, including falling and freezing, and often more marked postural tremor. Patients with vascular parkinsonism warrant a trial of levodopa but are generally less responsive than patients with idiopathic PD. The mainstay of treatment is physical therapy. To reduce the risk of future events, patients should be treated aggressively for reversible risk factors and receive antiplatelet agents.
Numerous agents block dopaminergic neurotransmission and produce parkinsonian symptoms. Classical antipsychotics used in schizophrenia, such as haloperidol, thioridazine, and perphenazine, work by blocking dopamine receptors. A predictable side effect of these medicines is the development of tremor, rigidity, and slowness of movement, indistinguishable from idiopathic PD. Elderly patients may develop parkinsonian symptoms when treated with haloperidol, risperidone, or olanzapine for nocturnal delirium in the hospital (sundowning). In some cases, patients are discharged from the hospital on these agents without a clear understanding of their nature. Drug-induced parkinsonism usually produces bilateral symptoms. Treatment begins with discontinuing the offending agent, but in some cases the symptoms persist. When symptoms persist after several months and interfere with the patient’s functioning, cautious therapy with levodopa may be warranted. In this situation, it is likely that treatment with an antipsychotic unmasked underlying idiopathic PD.
If absolutely essential for the management of hospital-acquired psychosis or agitation, atypical antipsychotics with fewer parkinsonian side effects can be used, such as quetiapine and clozapine, starting at very low doses. (Patients on clozapine need to have frequent blood tests to monitor for agranulocytosis.)
Drug-induced parkinsonism may also be a side effect of antiemetic medications that block peripheral dopamine receptors, such as metoclopramide, promethazine, and prochlorperazine. These agents may cross the blood–brain barrier and lead to parkinsonism. As with the antipsychotics, these drugs may be prescribed too liberally to hospitalized patients, and even chronically to outpatients. An antiemetic with a different mechanism of action, such as ondansetron, may be an appropriate alternative.
Differential Diagnosis: Nonparkinsonian Disorders
Problems with walking in the older patient may have variety of causes. These include pain, orthopedic problems, arthritis of the foot and ankle, fear of falling, and peripheral neuropathy, in addition to the non–basal ganglia disorders discussed here.
A condition that frequently brings patients to the neurologist for evaluation for possible PD is essential tremor (ET). This was previously known as benign familial tremor, but it is not necessarily familial and is not benign for those who are impaired by it. In ET, tremor is more typically bilateral than unilateral, although it can be asymmetric. In contrast to the tremor of PD, it is absent at rest and brought on by action, such as pouring a drink, bringing a cup to the mouth, or writing. There is little stiffness or postural instability, although severe tremor can affect walking and balance. There may be tremor in the neck, causing head oscillations. Tremor is worsened by stress and improved by relaxation. Alcohol may reduce the symptoms.
|
The diagnosis is made on clinical grounds. About 50% of ET cases are familial; thus, the presence of an affected family member supports the diagnosis. If the tremor is not apparent on examination, the patient should be asked to do whatever brings the tremor out, such as pouring water from one cup to another or writing. Drawing a spiral is useful to bring out subtle tremor and to document tremor severity.
Reversible causes of tremor should be excluded, specifically hyperthyroidism and medications such as lithium, valproate, theophylline, cyclosporine, and caffeine. If tremor improves with alcohol intake, mild alcohol consumption may ease embarrassment during social occasions; care should be taken that the patient does not develop dependence on alcohol. The two first-line medications are primidone, an older anticonvulsant, and propranolol. Other antiepileptic medications, such as gabapentin, levetiracetam, and topiramate, have been tried, with varying success. Deep brain stimulation (DBS, surgical implantation of electrodes) in the motor nuclei of the thalamus is used in severe cases.
Normal pressure hydrocephalus (NPH) is characterized by cognitive dysfunction, urinary incontinence, and gait apraxia, presumably from impaired resorption of cerebrospinal fluid (CSF). The possibility of this diagnosis may be raised by family members of the parkinsonian patient who have heard of impressive cures following ventriculoperitoneal shunt placement. The apraxic gait in NPH is characterized by a wide base, with slow, short steps, and the patient has difficulty lifting each foot off the ground, as if glued to the floor (magnetic gait). Although the gaits of NPH and PD may be similar, patients with PD usually do not freeze with each step, and the stance is not widened. Patients with NPH do not have a masked face, stooped posture, or slowed movements.
The gait disorder in NPH is caused by dysfunction or destruction of the periventricular fibers from the leg region of the motor cortex. The diagnosis of NPH is ultimately clinical but is supported by neuroimaging demonstrating ventricular enlargement out of proportion to cortical atrophy, impaired CSF flow after injection of radionuclide tracers, and alleviation of symptoms with removal of large volumes of CSF by lumbar puncture. However, placebo effects can occur with this procedure, and care should be taken to avoid false-positive interpretations, especially as placement of a ventriculoperitoneal shunt is not a benign procedure.
Cerebellar dysfunction causes a wide-based, ataxic gait, distinct from the shuffling gait of PD. However, the basal ganglia may be involved in cerebellar disorders, and parkinsonian symptoms may be seen, for example, in some of the autosomal dominantly inherited spinocerebellar ataxias or in the cerebellar form of MSA. The diagnosis is supported by eye movement abnormalities and limb or truncal ataxia.
Narrowing of the cervical canal with spinal cord compression can cause spasticity that interferes with walking. This may be detected as a clasp-knife increase in tone of the lower extremities, as distinguished from the lead-pipe rigidity of PD (see below). Increased activity of the deep tendon reflexes at the knees and ankles, with positive Babinski signs, will help localize the lesion to the cervical spinal cord. Atrophy of the small muscles of the hands due to cervical radiculopathy can be a sign of cervical spine disease. Clinicians should also bear in mind that spinal stenosis may complicate PD, as abnormal posture may promote spinal degeneration from increased vertebral wear and tear.
Diagnosis
There is no definitive test for PD prior to autopsy. The diagnosis is clinical, based on the presence of major criteria as delineated by the United Kingdom Brain Bank, namely, bradykinesia with resting tremor, rigidity, or postural instability, not related to another known etiology. Nonmotor symptoms, such as depression, anxiety, impaired cognition, muscle aches, insomnia, daytime somnolence, impaired olfaction, and constipation, may precede the onset of motor symptoms (Figure 211-1).