Symptom/side effect
Associated with opioids
Associated with pain
Management/monitoring approach
Depression
X
X
Consider treating depression as first step and observe effect on pain
Anxiety
X
X
Consider treating anxiety as first step and observe effect on pain
Agitation
X
X
Consider referral to psychiatry to determine cause and most appropriate treatment of agitation
Mobility difficulty/falls
X
X
Falls risk should always be screened in the older adult with chronic pain. If balance impairment is evident, an assistive device should be recommended along with referral to physical therapy for instruction in proper use. If opioids are considered, education regarding the risk of falls is essential for all older adults. If opioids are considered for the older adult with baseline mobility impairment, the practitioner must refer to physical therapy in an effort to optimize balance prior to prescribing opioids
Delirium
X
X
Patients with dementia have a heightened risk of delirium with opioids and with pain. A cognitive function screen should be considered an essential vital sign for older adults
Constipation
X
Discussion about starting a stimulant laxative at the first sign of constipation should occur at the time that the opioid is prescribed
Urinary retention
X
Especially important to educate the older male with benign prostatic hypertrophy and baseline voiding symptoms about this risk
Respiratory depression
X
More common in high doses
Sleep disturbance
X
X
Although nocturnal pain may prompt prescription of an opioid at bedtime, patients should be educated about their potential deleterious impact on sleep
Diminished appetite
X
X
As with other symptoms, the patient should ascertain the relative risks and benefits
Increased utilization of health care resources
?
X
Our clinical experience suggests that drug-seeking behavior is unusual in older adults in the absence of poorly treated pain
Myth #6
Treatment of pain in older adults with dementia should be guided by the same basic principles as for those who are cognitively intact.
Reality #6
Older adults with dementia are not simply a cognitively impaired version of those who are cognitively intact. An evidence base to guide treatment of pain in older adults with dementia is lacking; there is no substitute for thoughtful implementation and critical observation of empirical interventions.
Discussion: Just as aging is associated with extreme heterogeneity in the deterioration of biological, psychological, and social reserves as well as physical function, so too is dementia a heterogeneous process. The most common form of dementia is Alzheimer’s disease (AD) and the vast majority of data regarding pain, and dementia applies to this condition.
A number of studies that have been done with pain-free older adults in the laboratory highlight that those with Alzheimer’s disease have altered pain processing as compared with cognitively intact individuals. Functional brain imaging suggests that those with AD experience enhanced attention to painful stimuli as compared to those without AD [72]. Others have demonstrated that AD patients self-report pain intensity of acute stimuli (e.g., pressure, venipuncture) similar to that of cognitively intact individuals, but that their facial expressions associated with these stimuli are more exaggerated and nonspecific [73, 74]. Data also suggest that other behavioral manifestations of pain, such as guarding, bracing, and rubbing, also may be nonspecific in those with AD [75], that is, these “pain” behaviors may be an expression of the disordered movement that occurs in association with dementia, even in the absence of pain. Additional research in this area is clearly needed so that pain can be accurately detected in patients who have dementia and others with communication impairment.
Evidence also suggests that older adults with dementia may have blunted treatment expectancy [76]. It has been well-established in the pain literature that treatment expectancy is synergistic with pharmacodynamic analgesic efficacy [77, 78]. That is, the absence of belief in treatment efficacy negatively impacts treatment outcomes, even in those who are cognitively intact. If, in fact, patients with dementia have reduced treatment expectancy, these individuals may require larger analgesic doses to achieve desirable treatment outcomes. The reader should be aware that controlled studies of this hypothesis have never been undertaken and are needed. Until scientific evidence exists, the practitioner should be aware of the differences in pain processing between older adults with and without dementia and approach treatment prescribing accordingly.
Application to Clinical Practice
The key to optimizing treatment outcomes for older adults with chronic pain is to start with comprehensive assessment. The purpose of this assessment is threefold: (1) to identify all treatment targets, (2) to establish the patient’s unique pain signature that should be used to determine the efficacy of treatment, and (3) to identify key comorbidities that could constrain various treatment options. Table 19.2 outlines the essential components of a comprehensive history [79] and physical examination for the older adult with chronic pain that is designed to address each of these three goals.
Table 19.2
History and physical examination for the older adult with persistent pain: the essentials
History [79] |
Answers to the following questions will help to ascertain the older adult’s pain signature and, therefore, key treatment outcomes. |
1. How strong is your pain (right now, worst/average over past week)? |
2. How many days over the past week have you been unable to do what you would like to do because of your pain? |
3. Over the past week, how often has pain interfered with your ability to take care of yourself, for example, with bathing, eating, dressing, and going to the toilet? |
4. Over the past week, how often has pain interfered with your ability to take care of your home-related chores such as going grocery shopping, preparing meals, paying bills, and driving? |
5. How often do you participate in pleasurable activities such as hobbies, socializing with friends, and travel? Over the past week, how often has pain interfered with these activities? |
6. How often do you do some type of exercise? Over the past week, how often has pain interfered with your ability to exercise? |
7. Does pain interfere with your ability to think clearly? |
8. Does pain interfere with your appetite? Have you lost weight? |
9. Does pain interfere with your sleep? How often over the past week? |
10. Has pain interfered with your energy, mood, personality, or relationships with other people? |
11. Over the past week, how often have you taken pain medications? |
12. How would you rate your health at the present time? Excellent, good, fair, poor, or bad? |
Past history/review of systems: This portion of the history will identify key medical, psychological, and social comorbidities that may impact treatment response. |
Medical comorbidities | Relationship to treatment |
|---|---|
Constipation | If present at baseline, a stimulant laxative should be prescribed (e.g., senna) at the same time that an opioid is started |
Lower extremity edema | May be exacerbated by a nonsteroidal anti-inflammatory drug |
Hypertension Congestive heart failure Peptic ulcer disease Renal insufficiency | Gabapentin and pregabalin can contribute to lower extremity edema |
Obesity | Some medications may contribute to weight gain, such as gabapentin, pregabalin, and tricyclic antidepressants |
Sleep disturbance | While pain may disrupt sleep, opioids are also associated with disruption in sleep architecture |
Difficulty walking/falls | While pain itself can contribute to weakness, difficulty walking, and falls, older adults can have mobility difficulty independent of pain. In these individuals, care must be taken to avoid medications that can themselves contribute to mobility impairment, for example, opioids, pregabalin, gabapentin, and tricyclic antidepressants |
Memory loss | As noted in the text, pain itself can cause decrements in multiple domains of neuropsychological performance. With effective pain treatment, memory may improve. Practitioners must be aware, however, that many pain medications may contribute to confusion, for example, opioids, pregabalin, gabapentin, tricyclic antidepressants, and others (see Tables 19.4 and 19.5) |
Psychological factors | |
Depression | Untreated depression and/or anxiety can impair top-down inhibition; thus, the older adult with comorbid depression and/or anxiety must be treated for these disorders as part of pain treatment |
Anxiety | |
Coping skills | Poor coping skills (e.g., tendency to catastrophize) can inhibit the efficacy of pain treatment. While most cognitively intact older adults seem to cope well with chronic pain, the minority who do not should be referred for cognitive behavioral therapy as a part of pain treatment |
Self-efficacy | Physical therapy reduces fear avoidance beliefs (i.e., fear of moving because of concerns about exacerbating pain) in older adults [57]. Older adults with a history of falls may exhibit fear of falling, may have low confidence in mobility, and may have low self-efficacy (i.e., lack of confidence in their ability to engage in certain behaviors to affect desired outcomes). For these individuals, referral to a pain psychologist and physical therapist should be part of pain treatment |
Confidence in mobility | |
Fear of movement | |
Treatment expectancy | Treatment expectancy must be established at the outset of pain evaluation. Patients who believe that treatment will work will likely improve (i.e., placebo effect). Those who believe that treatment will not work will likely not improve (i.e., nocebo effect) |
Social factors | |
Social/caregiver support | Social isolation can interfere with the older adult’s ability to distract themselves from their pain and, therefore, intensify their pain experience. This may be especially problematic for the older adult with dementia. |
Financial status | The practitioner should always consider the older adult’s financial resources when prescribing treatments. |
Physical examination | |
1. Vital signs | |
(a) Cognitive function Mini-Cog [92, 93]: Examiner gives the patient three unrelated words to remember. Then, she/he gives the patient a blank piece of paper and asks them to draw a clock with the hands pointing to a specific time. Then, the patient is asked to recall the three words. Patients who are able to recall all three words have a low likelihood of dementia. Those who recall zero words have a high likelihood of dementia. For those who recall 1–2 words, the examiner should assess the accuracy of the clock-drawing test. If there are gross errors, the patient should be referred for evaluation of possible dementia. (b) Mobility (c) Traditional vital signs | |
2. Functional performance | |
(a) Balance Modified postural stress test ([94]; see Fig. 19.4): Examiner stands behind the patient with hands on sides of pelvis and states, “I am going to pull you backwards gently and try to throw you off balance…Do not let me…Are you ready?” Then, the examiner pulls the patient toward himself gently. If the patient is able to resist easily, try pulling a little more forcefully and observe response. The older adult whose balance is easily perturbed has decreased postural control and may be at heightened risk for falls. (b) Basic functional tasks – chair rise, ability to pick up object from floor, ability to place hands behind neck and waist (movements needed for dressing), and manual dexterity (e.g., ability to button and unbutton clothing, tie shoes) | |
3. Comprehensive identification of pain comorbidities | |
(a) Knee/hip arthritis in patients with low back pain (b) Shoulder disease in those with neck/upper back pain (c) Myofascial pain in all patients, including those with neuropathic pain [51] | |
4. Comprehensive routine physical examination | |
Below is a series of real cases that actualize how to integrate principles of aging into the practice of pain medicine and illustrate how to comprehensively identify treatment targets, establish the older adult’s pain signature (i.e., the way(s) that the patient manifests pain such as reduced appetite, difficulty walking, and confusion) [79], and identify potentially limiting comorbidities.
Case 1
An 82-year-old woman presented with low back pain for many years that had started insidiously and had lead to increasing functional limitations. She reported 7–8/10 sharp/burning daily pain that she experienced bilaterally, below the waist, and was worsened by standing, lifting, walking, and bending. There were no red flags. She had undergone numerous treatments without benefit including acupuncture, chiropractic, traction, physical therapy, aqua therapy, multiple epidural corticosteroid injections, and inpatient pain rehabilitation. She took prn naproxen for pain relief. Musculoskeletal examination revealed mild kyphoscoliosis, tenderness to palpation of both sacroiliac regions, and bilateral piriformis taut bands and trigger points. Neurological examination revealed symmetrical reflexes, 5/5 strength throughout, shortened stride length, and an anxious affect. The initial working diagnoses were (1) sacroiliac joint syndrome, (2) myofascial pain, and (3) anxiety for which physical therapy, sacroiliac joint injections, and gabapentin were prescribed.
One month later, she had experienced no pain reduction or functional improvement. A more detailed history uncovered the development over the past year of change in her voice (softening), handwriting (smaller), posture (increased forward flexion), and facial expression (less animated). A more detailed physical examination uncovered mild cogwheeling of her right arm. A neurology consultation was obtained to address the possibility of Parkinson’s disease. The consultant felt that there were “no full-blown Parkinsonian signs or symptoms,” but the presence of her masked facies, diminished blink, minimal asymmetrical cogwheeling, Myerson’s sign, and tendency to retropulse prompted a trial of levodopa/carbidopa 25/100 bid.
One month later, the patient reported average 4/10 pain (∼50 % reduction from baseline), improved posture, and balance as well as walking capacity and flexibility.
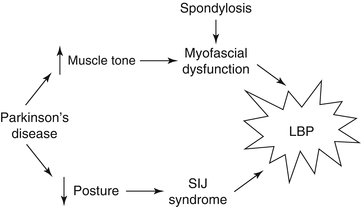
Discussion: The synthesis of this case is presented in Fig. 19.1. The treatment targets for this patient were her Parkinson’s disease and her myofascial pain. Her pain signature was comprised primarily of decreased physical function. Her impaired gait was also the primary comorbidity of concern. This placed her at heightened risk of falls. Had opioids been prescribed, the practitioner would have had to be especially vigilant for worsening mobility. Prior to prescribing such medications, the patient would have had to be educated about the risk of falls and hip fracture.
This case highlights the fact that PD is not infrequently associated with pain. Forty to fifty percent of patients with PD have pain that is not explained by other obviously painful disorders [80, 81]. Fifteen percent have pain as their presenting symptom (e.g., unilateral shoulder pain) [82]. Twenty-five percent have pain that precedes motor symptoms [83]. Patients may report muscle cramps or tightness, typically in the neck, paraspinal, or calf muscles; painful dystonias; joint pain; neuropathic pain; or less commonly, generalized pain [84]. Oral and genital pain syndromes that are similar to symptoms occurring in patients with tardive dystonia and akathisia from neuroleptics also have been described in patients with PD [85, 86]. The underlying pathogenesis of pain in PD can be central, peripheral, or mixed. Sensory thresholds to experimentally delivered painful stimuli are reduced in PD [87]. Unlike peripherally generated pain, such as that experienced by our patient, central PD pain that is associated with abnormal nociceptive input processing is not affected by dopamine administration [88].
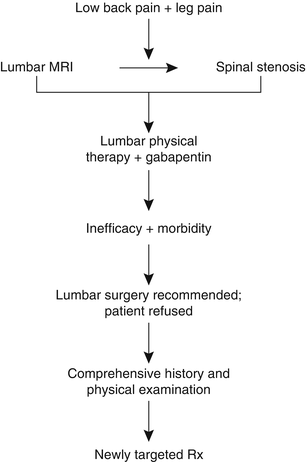
Perhaps most importantly, this case illustrates that successful treatment of the older adult with low back pain requires identifying the proper treatment targets (Parkinson’s disease [PD]) rather than simply treating the weak link (axial spondylosis). Had this patient elected to go forward with spinal surgery, the likely outcome, as compared with the actual outcome, is depicted in Fig. 19.2. In this figure, the “existing approach” represents common practice and the “proposed approach” is what we recommend.
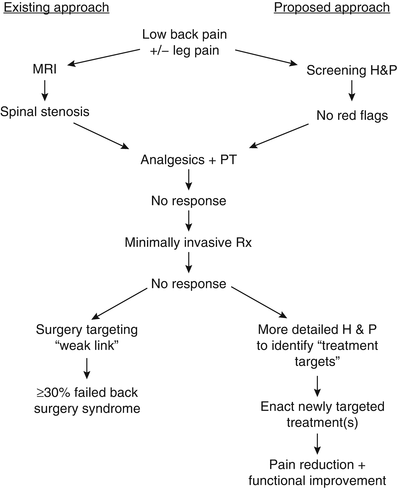

Fig. 19.2
A comparison of two approaches for the management of older adults with low back +/− leg pain. The approach commonly used (existing approach) focuses on imaging to direct treatment. Because the predictive value of abnormal imaging has not been critically examined in older adults and because abnormalities occur commonly, with or without pain, this approach frequently results in failed treatment. The proposed approach relies on a comprehensive history and physical examination to guide treatment that often targets multiple pain contributors. H&P history and physical examination, MRI magnetic resonance imaging, PT physical therapy
Case 2
An 82-year-old woman presented with low back pain and right leg pain for two years with documented central canal stenosis on MRI. She had worked full time in a dress shop and was forced to retire 2 years ago because the company was downsizing. She said that her pain started at that time and had gotten progressively more severe. Her pain was made worse by prolonged standing or walking, and she was having increasing difficulty performing heavy housework. Her pain was made better with rest and heat application. She denied fever, chills, weight loss, and change in her bowels or bladder function. She reported poor balance and multiple near falls at home. She lived alone. She was becoming increasingly fearful of leaving her home. Medications at the time of presentation, all of which had been prescribed to treat her pain and pain-associated anxiety, included gabapentin, oxycodone CR, celecoxib, tramadol/acetaminophen, olanzapine, escitalopram, and lorazepam. Physical examination revealed poor balance, dementia (memory problems and very impaired clock-drawing test) [89], kyphoscoliosis, and tenderness of the right sacroiliac joint/lumbar paraspinal musculature/tensor fasciae latae/iliotibial band. Because of extreme guarding behavior, strength testing was invalid.
Because of polypharmacy, high falls risk, and social isolation, the patient was admitted to a nursing home for detoxification. All of her medications were discontinued with the exception of regularly scheduled acetaminophen and prn tramadol. She reported minimal pain and her balance improved markedly. It was recommended that her family strongly consider placing her in an assisted living facility. They chose to seek other opinions from pain practitioners. Immediately following discharge, the patient’s pain complaints escalated and multiple other pain regimens were attempted including a morphine pump trial, all of which failed. She was eventually placed in an assisted living facility where she did well.
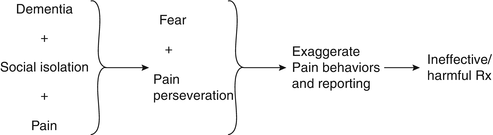
Discussion: The synthesis of this case is presented in Fig. 19.3. As noted earlier in this chapter, to prescribe effective treatment, the practitioner must differentiate the weak link from the treatment target(s). In this case, chronic pain was the weak link and fear/social isolation the treatment targets. Her pain signature consisted of pain perseveration and significant utilization of health care resources. The main potentially treatment-limiting comorbidities were her dementia and balance frailty.
One of the first discussions that we have with patients in chronic pain revolves around treatment expectations. Specifically, patients with chronic pain need to understand that it is realistic to expect partial but not complete pain relief. Treatment of the older adult with dementia is complicated by the fact that information provided in treatment counseling sessions may not be remembered and ongoing reinforcement may be necessary. Such reinforcement is often successful when the patient has an involved and supportive caregiver (and one who does not catastrophize about the patient’s pain) and health care providers who are willing to communicate a consistent message. In this patient’s case, inconsistent messages were delivered (i.e., although it was clear that the patient’s fear and social isolation in the setting of dementia were primarily responsible for her suffering, the patient’s family insisted that her pain was responsible and more aggressive pain treatment was sought).
While many patients with dementia can report pain reliably [90, 91], the meaning of these reports must be ascertained in order to prescribe effective treatment. Is the patient’s pain reporting a manifestation of perseveration (that occurs not uncommonly in patients with dementia)? Or, is the patient’s pain reporting a more general signal of distress? Or, is the patient’s pain reporting an indication of pain-related suffering? If there is pain-related suffering, then pain-specific treatment must be implemented. In the case of our patient, her pain reporting appeared to be a manifestation of both perseveration and a more general signal of distress (i.e., anxiety surrounding social isolation and dementia). Thus, while treatment did involve analgesics, providing a supportive environment was the primary therapeutic element.
This case highlights the need to screen for dementia at the time of the initial history and physical examination. One of the most efficient and effective screening tools is the Mini-Cog, described in Table 19.2 [92, 93]. It takes no more than 2–3 min to perform. If this testing uncovers the possibility of dementia, the patient should be referred to a geriatrician for further evaluation. Older adults with and without dementia often have mobility difficulty and a risk of falling; thus, a balance screen should also be included as part of the baseline assessment. A modified postural stress test [94] can readily be done in the office and is described in Table 19.2 and shown in Fig. 19.4. If this test reveals poor balance, a referral to physical therapy should precede any intervention that could further impair balance (e.g., opioid prescription).
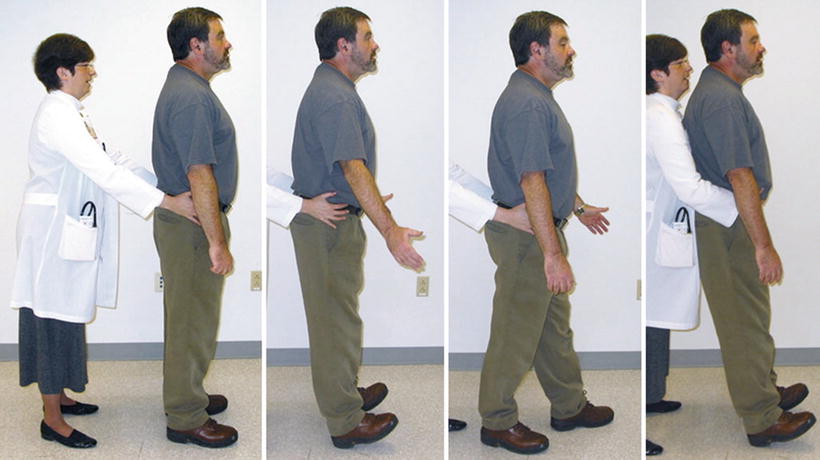

Fig. 19.4
Modified postural stress test. The highest level postural response (i.e., associated with the best balance) is shown on the far left, where there is no obvious movement in response to attempted perturbation. The lowest level “timber response” is shown on the right, where the patient makes no effort to recover upright stance. This response is highly unusual and typically indicates severe supratentorial dysfunction. The middle two photographs depict intermediary responses.
Case 3
An 85-year-old man with advanced Alzheimer’s disease presented, along with his wife of 60 years and their daughter, for treatment recommendations to address “persistent reporting of pain” in his lower back. His primary care provider was concerned because the patient’s pain ratings had not changed despite numerous analgesic prescriptions. Most recently, he had been prescribed fentanyl that had been titrated to a dosage of 100 mcg/72 h and resulted in hospitalization because the patient became semicomatose. When the dosage was decreased to 50 mcg/72 h, his mental status returned to baseline and he continued to report pain so a pain clinic consult was requested.
At the time of the evaluation, he was sitting in a wheelchair, appeared very comfortable, smiled throughout most of the interview, and had no pain complaints. His history was unreliable because of advanced dementia. His wife reported that the patient had low back pain for many years. She was asked, “Do you think your husband is suffering from his pain, or is he just talking about it?” Without hesitation, she replied, “Oh…he’s just talking about it.” Together, we decided that the most appropriate treatment would include tapering him off the fentanyl and have him participate in a local day care program for socialization and distraction. His family was educated about the fact that patients with chronic low back pain cannot be made pain-free and that the main goal of treatment is preservation and/or improvement in function to the extent possible. She understood and fully supported the plan.
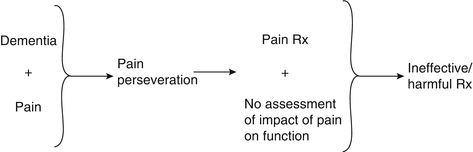
Discussion: The synthesis of this case is presented in Fig. 19.5 and reinforces the complexities of pain evaluation and management in older adults with dementia. In this patient, chronic pain was the weak link and pain perseveration the treatment target. Pain perseveration was also the major component of his pain signature. The main potentially treatment-limiting comorbidity was his dementia (i.e., increased risk of falls and/or delirium with opioids). As opposed to the patient in Case 2 whose pain reporting reflected pain perseveration as a general signal of distress, this patient’s pain reporting was a simple representation of pain perseveration that was treated with distraction. Often, this type of perseveration behavior in older adults with dementia is more a problem for the caregiver (i.e., it is stressful to observe the perceived suffering of a loved one, contributing to caregiver burden) than for the patient, and treatment strategies should keep this in mind.
This case also highlights the importance of patient-centered or patient/caregiver-centered decision making. In busy office practices, it may be difficult to take the extra time required to engage in these discussions. Not doing so, however, may lead to unnecessary morbidity, as was the case with this patient.
Case 4
A 67-year-old man presented with low back and left leg pain for 10 months. He had injured his back nearly 50 years earlier associated with heavy lifting. He was treated conservatively and his pain abated within 1–2 months. Ten months ago, he experienced the insidious onset of sharp/burning pain in his left lower back with occasional radiation to the left leg (lateral aspect) that was getting progressively more severe. He reported occasional weakness and numbness of the left leg and progressively more restricted walking tolerance. At the time of presentation, he ambulated with a walking stick and could go one-half block before he had to stop because of pain. He reported multiple falls because his leg “gave way.” His pain was worsened by lying prone and trying to straighten his leg while lying supine. It was made better by lying on his side and assuming a fetal position. He denied fever, chills, or change in his bowels/bladder.
A lumbar MRI performed 2 months following the onset of his pain revealed diffuse lumbar spondylosis and moderate central canal stenosis. Treatment had included (1) physical therapy for lumbar spinal stenosis that resulted in no improvement in his pain or function; (2) tramadol that was ineffective; (3) gabapentin that caused nausea, vomiting, and a 15-lb weight loss; and (4) hydrocodone/acetaminophen that was associated with moderate pain relief. Spinal surgery was recommended, but he declined. His only significant medical comorbidity was hypertension.
Notable on physical examination was blood pressure 178/96, ¾ in. leg length discrepancy, mild scoliosis, mild left piriformis tenderness, and an antalgic gait with favoring of the left leg. His gait was slow but steady when performed with his walking stick. Examination of the left hip revealed <15° painful internal rotation. Right hip exam was normal. Neurological exam revealed symmetrical lower extremity reflexes and 5/5 strength throughout with the exception of the left hip flexors and left quadriceps that were 4/5. When the patient was lying supine and asked to raise his left leg, he did so by picking it up with his hands. Hip x-rays revealed marked joint space narrowing of the superior and inferior aspects on the left and no abnormalities on the right. Based on these findings, he was instructed to continue regularly scheduled hydrocodone/acetaminophen and he was referred to physical therapy specifically directed toward the left hip. If these strategies are ineffective, he will be referred for intra-articular hip injection. If he is refractory to all noninvasive and minimally invasive treatments, he will be referred for consideration of a total hip replacement.
Discussion: The synthesis of this case is shown in Fig. 19.6. The treatment target was his hip osteoarthritis. His pain signature consisted of severe self-reported pain and difficulty walking. His significant comorbidities included hypertension and difficulty walking/falls. Because his symptoms were initially attributed to the lumbar spine, he underwent an unnecessary lumbar MRI and was prescribed medications that resulted in significant adverse events, physical therapy that was ineffective, and a referral for spinal surgery that would likely not have relieved the “pain generator.”
This case is presented to highlight the important contribution of hip osteoarthritis to low back pain. The hip-spine syndrome was first described in 1983 and refers to symptoms that exist in the setting of concurrent degenerative pathology in the hip and spine [95]. Three types of hip-spine syndrome were postulated: (1) simple when history and physical examination clearly indicate whether the hip or the spine is the primary source of pain; (2) complex, when both the hip and the spine are responsible for pain; these cases are said to require ancillary investigations such as nerve root infiltration and intra-articular blocks of the hip joint to disentangle the primary source of pain; and (3) secondary, when altered hip function (e.g., flexion deformity with advanced OA) directly changes spinal biomechanics that cause low back pain. The contribution of hip OA to CLBP also is supported by more recent data. Specifically, total hip replacement surgery for patients with severe hip pain and advanced OA on x-ray reduces low back pain and improves overall spine function [96]. In patients with low back pain, diminished hip range of motion predicts poor outcomes following spinal manipulation [97] and after lumbar percutaneous electrical nerve stimulation (unpublished data). Preliminary data suggest that patients with self-reported hip OA respond less favorably to decompressive laminectomy for the treatment of lumbar spinal stenosis (LSS) than those without hip OA [98].
It is likely that many older adults have hip-spine syndrome that is both complex and secondary in which both the hip and spine are pain generators, but altered hip function causes abnormal spinal biomechanics and low back pain, that is, altered hip function adds insult to injury. Although severe hip flexion deformity may be absent, we hypothesize that underlying lumbar spondylosis makes the lower back vulnerable and, therefore, more modest alterations in hip function may be needed to cause low back pain. So, the lumbar spine is the weak link and the hip is the treatment target. Well-controlled studies are needed to test this hypothesis. Until definitive answers are available, practitioners must approach the older adult with low back and/or leg pain using a broad perspective to avoid unnecessary “diagnostics” and misguided/potentially harmful treatments. Table 19.3 highlights key history and physical examination differences between pain generated by lumbosacral degeneration and that associated with hip OA. It should be noted that hip x-rays alone cannot be used to make a diagnosis of clinically meaningful hip OA. Fewer than 50 % of patients with radiographic evidence of hip OA report pain [11]. A definitive diagnosis of hip OA should be based on a combination of clinical examination and x-ray findings [99, 100]. Thus, careful examination of the hip should be a routine part of evaluating all older adults who present with low back and/or leg pain.
Table 19.3
Differentiation of lumbosacral from hip-generated low back/leg pain
Feature | Lumbosacral | Hip | Comments |
|---|---|---|---|
Pain location | Above pelvis; if comorbid spinal stenosis, pain may involve buttocks and/or legs | Most common referral patterns are buttocks, groin, and thigh | If sacroiliac joint syndrome (SIJS) complicates hip disease, SI pain can coexist with buttocks/groin/thigh pain |
Leg pain | Present if comorbid spinal stenosis or knee/hip disease | Often | Radiculopathy pain typically extends the entire leg. Although hip pain can be referred to the lower leg and/or foot, most commonly it involves the buttocks, groin, and thigh |
Groin pain | Absent | Often | SI pain can be referred to the groin, so if SIJS complicates lumbosacral pathology, groin pain can occur |
Movements that aggravate pain | Spinal extension | Leg extension | If comorbid SIJS, side lying and/or flexion may worsen pain |
Hip internal rotation | |||
Movements that alleviate pain | Spinal flexion | Hip flexion | If spine and hip disease co-occur, response to movement patterns may be atypical |
Hip external rotation | |||
Posture | Spinal flexion | Leans forward, with flexion at the hip | When spine or hip disease is mild, there may be no obvious postural abnormalities |
Associated symptoms | Paresthesias, radiculopathic pain, lower extremity weakness | Lower extremity weakness | If spine and hip disease co-occur, symptoms can overlap |
X-ray findings | Poor predictive validity for pain | Poor predictive validity for pain | |
Treatment Guidelines
The overarching goal of treatment for the older adult with chronic pain is to optimize function and quality of life while minimizing the potential for adverse effects associated with treatment. To accomplish this goal, an integrative stepped-care approach that combines non-pharmacological and pharmacological modalities is recommended. Specific recommendations for treating older adults with nociceptive pain, neuropathic pain, and widespread pain are provided below.
Nociceptive Pain
Figure 19.7 depicts an integrative stepped-care approach for the treatment of nociceptive pain. Topical preparations, cognitive behavioral therapy, interdisciplinary pain treatment, and complementary and alternative modalities (CAM) may be used at any step, either alone or in combination. The individual steps shown in Fig. 19.7 are arranged from treatments associated with relatively low risk (step 1) to those associated with high risk (step 7).
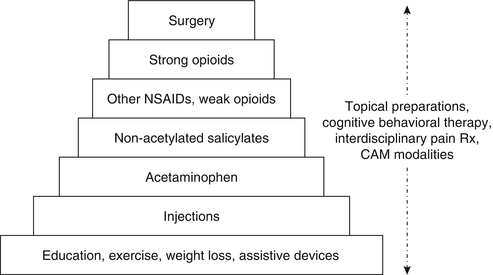

Fig. 19.7
Stepped-care approach for the treatment of nociceptive pain. CAM complementary and alternative medicine, NSAIDs nonsteroidal anti-inflammatory drugs (Reprinted from: Weiner and Cayea [178], with permission from Debra Weiner and IASP Press)
At the foundation of treatment are education, weight loss, exercise, and other physical therapy approaches (including assistive devices). Sometimes, these approaches are alone sufficient to accomplish desired outcomes. For the older adult with fibromyalgia who is capable of participating in aerobic exercise, no further treatment may be needed. For the patient with kyphosis related to vertebral compression fractures and associated lumbar strain, a four-wheeled walker often is very effective for reducing pain and improving mobility. Education should be targeted at ensuring realistic treatment expectations (i.e., pain reduction but not elimination and improved function despite the persistence of pain) and quelling any pain-associated fears (e.g., becoming crippled and/or losing independence because of pain, having cancer associated with pain).
To avoid risks associated with systemic medication, injections should be considered for the older adult with pain in one or two joints, for example, knee osteoarthritis (OA). Trigger point injections can be an effective adjunct for treating myofascial pain syndromes [101]. There is no strong evidence to guide the prescription of spinal injections for older adults with chronic low back pain (CLBP). In general, injection therapies should be viewed as a tool to enhance compliance with rehabilitation efforts, which represent the mainstay of nociceptive pain treatment. For older adults with diabetes mellitus, patients should be instructed to monitor their blood sugar carefully following corticosteroid injections.
Systemic pharmacologic treatment of mild to moderate nociceptive pain should start with regularly scheduled acetaminophen because of its relatively safe side effect profile and few drug-drug or drug-disease interactions. Acetaminophen exerts its analgesic effect by weak, reversible, nonspecific cyclooxygenase inhibition, and, therefore, prostaglandin synthesis. It has no anti-inflammatory or antiplatelet effect and uncommonly causes gastrointestinal (GI) bleeding or nephrotoxicity [65]. An overdose of 10 g can cause liver failure and death. Hepatic injury can occur with lower doses when the patient drinks alcohol heavily or is taking hepatic enzyme inducing medications (e.g., rifampin, carbamazepine, phenytoin, phenobarbital). Preexisting liver disease, malnourishment, fasting, or dehydration can also increase the risk of liver injury. Table 19.4 provides dosing guidelines, pharmacokinetics, key drug-drug and drug-disease interactions, and important adverse effects associated with acetaminophen and other medications used for nociceptive pain. To avoid breakthrough pain, it is important to dose analgesics around the clock [102].
Table 19.4
Oral analgesics for nociceptive pain
Medication class | Medication | Recommended dosing | Pharmacokinetics | Key drug-drug interactions | Key drug-disease interactions | Important adverse effects |
|---|---|---|---|---|---|---|
Other analgesic | Acetaminophen | 325–1,000 mg q 4–6 h Maximum daily dose 4,000 mg | Metabolized via glucuronidation Clearance may be reduced in frail older adults | Hepatic injury can occur with modest doses when concomitant use of hepatic enzyme inducing medications (e.g., rifampin, carbamazepine, phenytoin, phenobarbital). | None | Hepatic necrosis with acute 10 g ingestion or chronic use of >4 g/day. Increased toxicity from chronic use occurs with heavy alcohol use, malnourishment, pre-exiting liver disease – decrease maximum daily dose to 2 g Nephrotoxicity (dose dependent) |
Non-acetylated salicylates | Salsalate | 500–750 mg bid | Metabolized by hydrolysis to salicylate; also metabolized via glucuronidation | No significant drug-drug interactions | See other NSAIDs below | Does not interfere with platelet function; GI bleeding rare |
Maximum dose 3,000 mg/day | ||||||
Other NSAIDs | Ibuprofen Naproxen | 400 mg tid-qid 250–500 mg bid | CYP2C9/19 CYP2C9 and CYP1A2; clearance significantly reduced with advanced age | Concomitant use of NSAIDs with diuretics and antihypertensives may decrease their effectiveness. Use with corticosteroids and/or warfarin increases the risk of peptic ulcer disease. Increases concentration of lithium and methotrexate | Use NSAIDs with caution in patients with chronic renal failure, heart failure, hypertension, and peptic ulcer disease history | Risk of GI bleeding increased in persons ≥ 60 years. Cognitive impairment possible with higher doses. These NSAIDs should be reserved for short-term use in the older adult. |
Cyclooxygenase (COX-2) inhibitor | Celecoxib | 100 mg bid | CYP2C9/19 | Same as NSAIDs above | Same as NSAIDs above | Because of relatively long half-life, naproxen should not be first choice for older adults. |
COX-2 inhibitor has less GI toxicity, but similar renal toxicity to other NSAIDS. | ||||||
Given long half-life and perhaps greater cardiac toxicity make it not a preferred agent for older adults. | ||||||
Weak opioids | Codeine Hydrocodone | 15–30 mg q 4–6 h 5–10 mg q 4–6 h (alone or in combination with acetaminophen) | Prodrug metabolized by CYP2D6 CYP2D6;not studied in older adults | Few clinically significant drug-drug interactions. Quinidine can inhibit the analgesic effect of codeine. | For all opioids, increased risk of falls in patients with dysmobility. May worsen or precipitate urinary retention when BPH present. Increased risk of delirium in those with dementia Codeine has active renal metabolites that can accumulate with advancing age and renal insufficiency. | Because of increased sensitivity to opioids older adults at greater risk for sedation, nausea, vomiting, constipation, urinary retention, respiratory depression, and cognitive impairment. |
Opiate receptor agonist/SNRI | Tramadol | Initiate at 25 mg qd. Increase by 25–50 mg daily in divided doses every 3–7 days as tolerated to max dose of 100 mg Four times a day (QID). Renal dosing 100 mg Twice a day (BID). | Prodrug metabolized by CYP2D4, 2B6 and 2D6. | Sedative medications and other opioids. Risk of serotonin syndrome in combination with SSRI and triptans. Seizure risk with MAOI. Rare reports of interaction with warfarin and digoxin. Quinidine can inhibit the analgesic effect. | Seizure disorder (avoid if history of seizures). | Seizures and orthostatic hypotension. Other side effects similar to traditional opioids including sedation, confusion, respiratory depression. |
Adjust dose with renal insufficiency; maximum dose 100 mg bid. | ||||||
Strong opioids | Oxycodone (short and long-acting) | Start with 5 mg (short acting) q 4–6 h; after 7 days, determine dose requirements, then convert to long acting. | CYP2D6 | As above | As above Oxycodone and morphine have active renal metabolites that can accumulate with advancing age and renal insufficiency. | As above Stong opioids to avoid in older adults: pentazocine, meperidine. |
Morphine (short and long-acting) | Start with 2.5 mg q 4 h and titrate by 2.5 mg increments q 7 days. Convert to long acting after dosing requirements determined. | Large first pass effect and high hepatic extraction ratio results in higher serum levels and decreased clearance; glucuronidation to active renally cleared metabolites. | ||||
Hydromorphone | Start with 2 mg q 4 h. Increase after 7 days if needed. | Glucuronidation; not studied in older adults | Dose of opioid required (for weak, strong, and tramadol) can be reduced by combining with a non-opioid agent such as acetaminophen. | |||
Fentanyl transdermal | Start with 12 mcg patch q 72 h. If ineffective after 1 week, increase to 25 mcg. 48 h dosing interval may be required.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|