Introduction
Pain has its uses! It tells us to avoid situations that can cause serious damage to our bodies. That information is essential but when patients suffer from chronic, continuing pain it no longer serves any useful purpose and is an unpleasant and aversive experience.
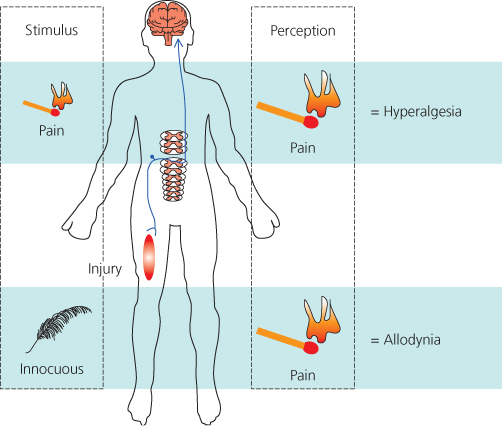
Chronic pain states arise following tissue damage or injury to the peripheral and or central nervous system and are broadly termed ‘inflammatory’ or ‘neuropathic’, respectively. Both inflammatory and neuropathic pain states are characterised by spontaneous pain, hyperalgesia (exaggerated pain) and allodynia (touch evoked pain) (Figure 2.1). These symptoms may encourage behavioural adjustments that promote repair and recovery by limiting contact with, for example, wounded tissue. However, if these symptoms persist beyond tissue healing, these chronic pain symptoms can be extremely debilitating and greatly reduce quality of life.
Figure 2.1 Chronic pain states arise following injury and are characterised by the symptoms of hyperalgesia (exaggerated pain), allodynia (touch-evoked pain) as well as continuing or spontaneous pain.
Chronic pain states transform dramatically the somatosensory nervous system, from one in which pain normally serves as a warning signal, promoting life and survival, to one in which pain is evoked by everyday activities and is counter-productive. In this chapter, those nervous system components that transduce and process sensory information are examined, how these components change or malfunction following injury is described and how these alterations are thought to produce chronic pain is explained.
Basic Pain Pathway
Sensory information is conveyed from the periphery to the central nervous system via primary sensory neurons. There are different types of sensory neurons (Table 2.1) but all have their cell bodies in the dorsal root ganglia (Figure 2.2).
Table 2.1 Some of the different types of peripheral nerve fibres*
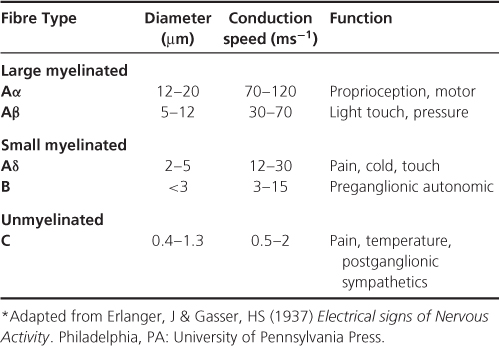
Figure 2.2 Basic somatosensory pathways. Sensory information is carried from the periphery to the spinal cord by primary sensory neurons, which have their cell bodies in dorsal root ganglia. Sensory information is then processed in the dorsal horn of the spinal cord before it is sent to the brain. There are also descending influences from the brain.
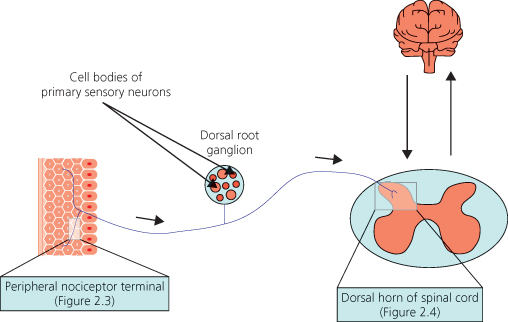
The pain-sensing primary sensory neurons, or ‘nociceptors’ have naked peripheral endings that terminate in the skin, mainly in the epidermal layer. These ‘peripheral nociceptor terminals’ possess an array of receptors or ion channels that transduce mechanical, thermal and chemical stimuli into neural signals (Figure 2.3a). Following sensory transduction, neural signals are then transmitted via primary sensory neurons to the dorsal horn of the spinal cord – the first stage of central processing of sensory input (Figure 2.4a).
Figure 2.3 Pain detection by the peripheral nociceptor terminal and its amplification (peripheral sensitisation) in chronic pain states. (a) Noxious stimuli are transduced into electrical signals by specific receptors and ion channels. Our knowledge of pain detection has been transformed by the discovery of the TRP (Transient Receptor Potential) channels that are thermo- and chemo-sensitive. It is not fully understood which receptor or receptors are responsible for transducing mechanical pain. (b) Following injury, damaged tissue cells and inflammatory cells release inflammatory mediators. These activate intracellular signalling pathways that modify transducer receptor and ion channel function, which increases the sensitivity of the peripheral nociceptor terminal to sensory stimuli.
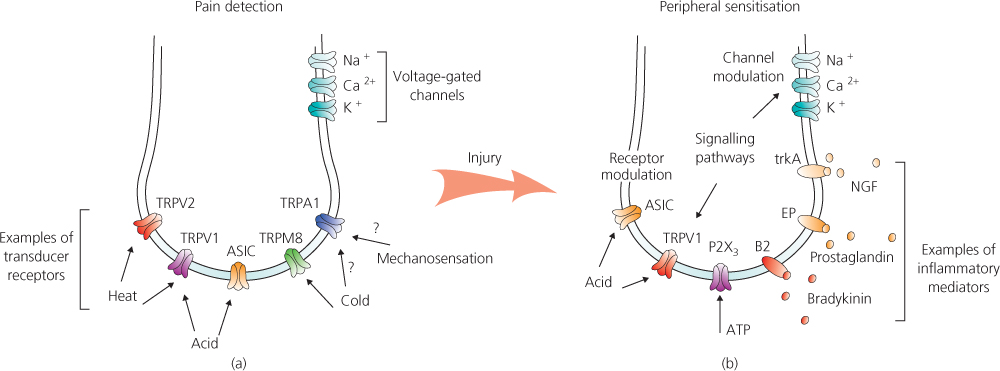
Figure 2.4 Representation of spinal cord processing of sensory input and its distortion (central sensitisation) in chronic pain states. (a) Incoming sensory information undergoes local and descending modulation in the spinal cord dorsal horn. (b) Dorsal horn sensory processing is distorted in chronic pain states. There is increased sensory input, increased local excitation, decreased local inhibition and altered descending control. Overall, this increases spinal cord excitability and amplifies responses to sensory input.
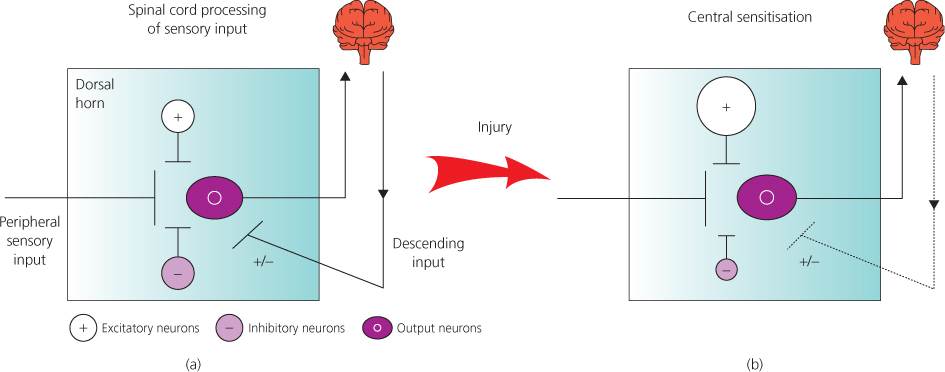
The processing of sensory information within the dorsal horn is complex, involving local excitatory and inhibitory influences, as well as descending modulation from the brain. Importantly, this dorsal horn sensory processing determines which sensory signals are sent to higher centres to be perceived, where they may also influence emotional and autonomic function.
Chronic Pain
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








