From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, MO, 2011, Elsevier. Copyright 1999, Chris Pasero, Margo McCaffery. Used with permission.
Some patients have “mixed pain,” which is a combination of nociceptive and neuropathic pain.10,11 For example, a patient may have nociceptive pain as a result of tumor growth and also report radiating sharp and shooting neuropathic pain if the tumor is pressing against a nerve plexus. Sickle cell pain is usually a combination of nociceptive pain from the clumping of sickled cells and resulting perfusion deficits and neuropathic pain from nerve ischemia.
Some painful conditions and syndromes are not easily categorized as either nociceptive or neuropathic. These are sometimes referred to as mixed pain syndromes and include fibromyalgia and some low back and myofascial pain.
Nociception and Analgesic Action Sites
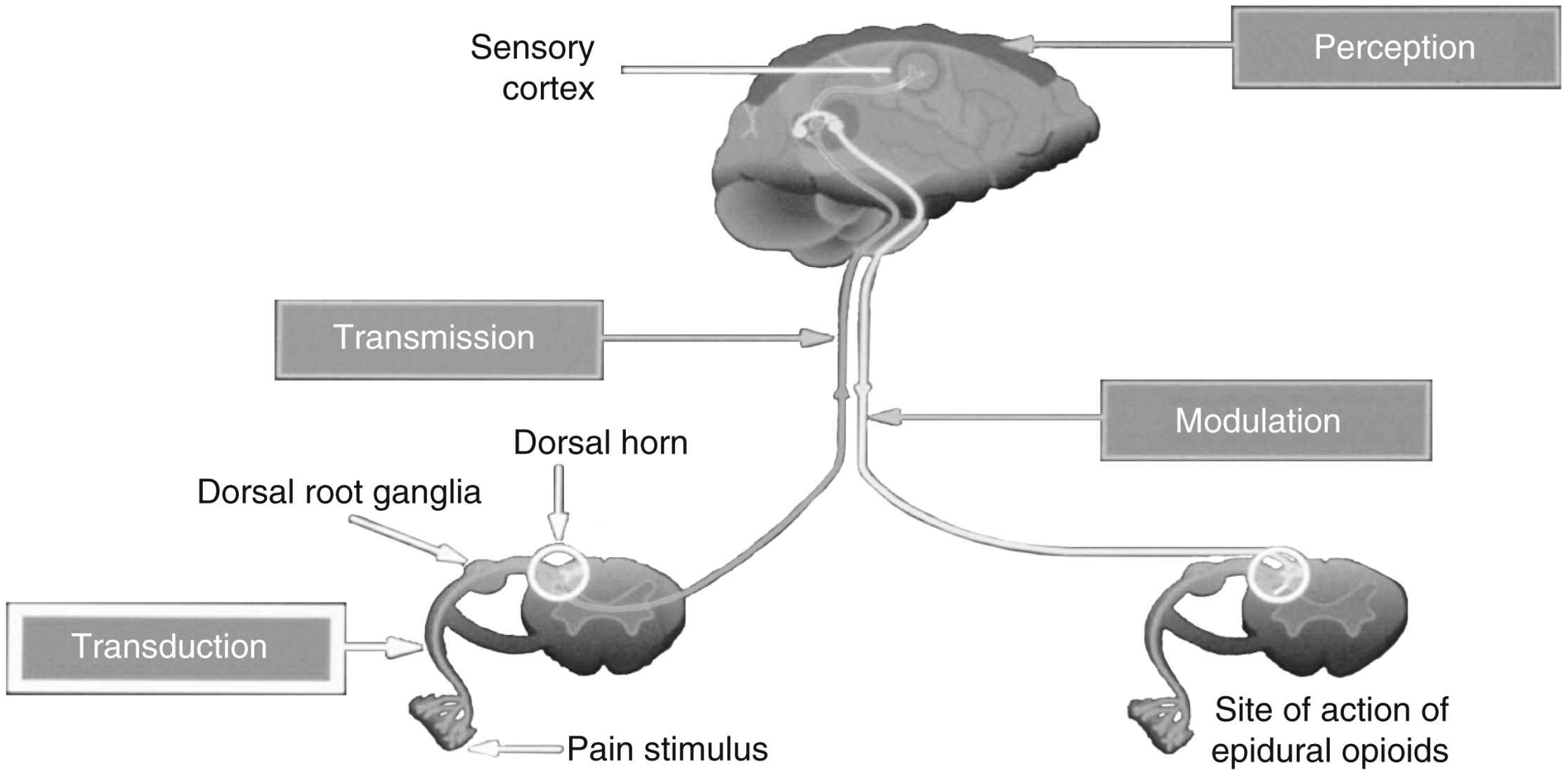
Nociception includes four specific processes: transduction, transmission, perception, and modulation. Fig. 31.1 illustrates these processes, and an overview of each follows.
Transduction
Transduction refers to the processes by which noxious stimuli activate primary afferent neurons called nociceptors, which are located throughout the body in skin, subcutaneous tissue, and visceral and somatic structures (see Fig. 31.1).8 These neurons have the ability to respond selectively to noxious stimuli generated as a result of tissue damage from mechanical (e.g., incision, tumor growth), thermal (e.g., burn, frostbite), chemical (e.g., toxins, chemotherapy), and infectious sources.12,13 Noxious stimuli cause the release of a number of excitatory compounds (e.g., serotonin, bradykinin, histamine, substance P, prostaglandins), which facilitate the movement of pain along the pain pathway.8 These substances are collectively referred to as inflammatory soup.13
Prostaglandins are a particularly important group of compounds that accompanies tissue injury and initiates inflammatory responses that increase tissue swelling and pain at the site of injury.14 They are formed when the enzyme phospholipase breaks down phospholipids into arachidonic acid, and arachidonic acid, in turn, is acted upon by the enzyme cyclooxygenase (COX) to produce prostaglandins (Fig. 31.2). The two best characterized isoenzymes of COX are COX-1 and COX-2; they have an important role in producing the effects of the nonopioid analgesics, which act peripherally and centrally to inhibit the COX isoenzymes. Nonsteroidal antiinflammatory drugs (NSAIDs) work primarily by blocking the formation of prostaglandins in the periphery. The nonselective NSAIDs, such as ibuprofen, naproxen, diclofenac, and ketorolac, inhibit both COX-1 and COX-2, while the COX-2-selective NSAIDs, such as celecoxib, inhibit just COX-2. As Fig. 31.2 illustrates, both types of NSAIDs produce antiinflammatory and pain relief through the inhibition of COX-2. Although the exact underlying mechanisms of action of acetaminophen continue to be investigated,15,16 acetaminophen is a known COX inhibitor that has minimal peripheral effect, is not antiinflammatory, and can both relieve pain and reduce fever by preventing the formation of prostaglandins in the CNS.8
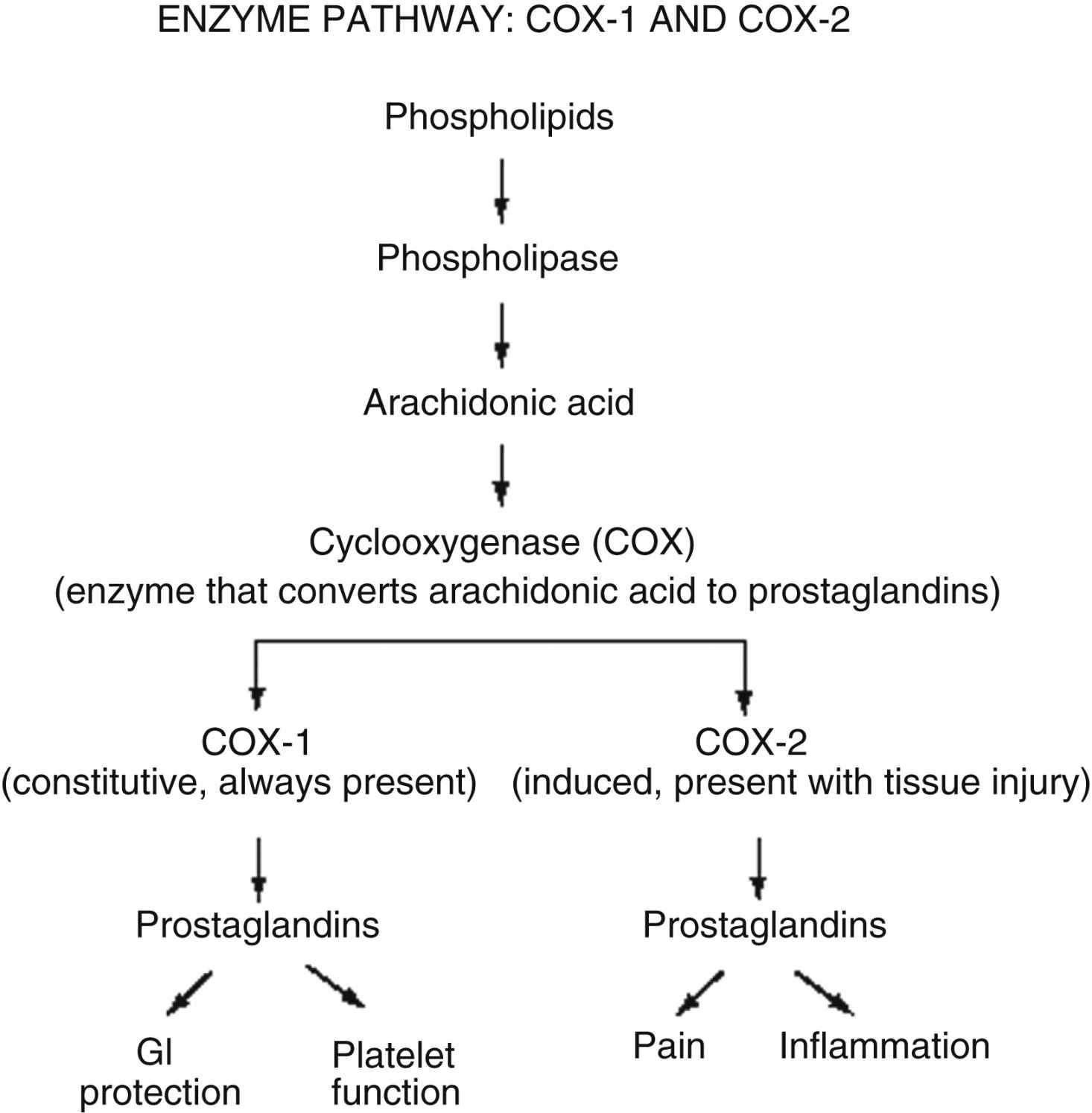
FIG. 31.2 Enzyme pathway: COX-1 and COX-2. GI, Gastrointestinal. (From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, MO, 2011, Elsevier. Copyright Pasero C, McCaffery M. Used with permission.)
Other types of analgesics work by partially blocking transduction as well. For example, sodium channels are closed and inactive at rest but undergo changes in response to membrane depolarization. Transient channel opening leads to an influx of sodium and subsequent nerve conduction.17 Local anesthetics are capable of blocking sodium channels and reducing the nerve’s ability to generate an action potential. Anticonvulsants also affect the flux of other ions, such as calcium and potassium, to reduce transduction and produce pain relief.
Transmission
Transmission is the second process involved in nociception. Effective transduction generates an action potential transmitted along the A-delta (δ) and C fibers.8 A-δ fibers are lightly myelinated and faster conducting than the unmyelinated C fibers. The endings of A-δ fibers detect thermal and mechanical injury and allow relatively quick localization of pain and a rapid reflex withdrawal from the painful stimulus. Unmyelinated C fibers are slow conductors and respond to mechanical, thermal, and chemical stimuli. They yield poorly localized, often aching or burning pain. A-beta (β) fibers are the largest of the fibers and do not normally transmit pain but do respond to touch, movement, and vibration.8
Afferent information passes through the cell body of the dorsal root ganglia (see Fig. 31.1), which lie outside of the spinal cord, to synapses in the dorsal horn of the spinal cord. An action potential is generated, and the impulse ascends up to the spinal cord and transmits information to the brain, where pain is perceived. Extensive modulation occurs in the dorsal horn via complex neurochemical mechanisms. The primary A-δ fibers and C fibers release a variety of transmitters including glutamate, neurokinin, and substance P. Glutamate binds to the N-methyl-D-aspartate (NMDA) receptor and promotes pain transmission. Ketamine is an NMDA receptor antagonist that provides pain relief by preventing glutamate from binding to the NMDA receptor sites. Endogenous and therapeutically administered opioids bind to opioid receptor sites in the dorsal horn to block substance P and thereby produce analgesia.8
Perception
The third broad process involved in nociception is perception. Perception, the result of the neural activity associated with transmission of noxious stimuli,8 involves the conscious awareness of pain and requires activation of higher brain structures for the occurrence of awareness, emotions, and drives associated with pain (see Fig. 31.1). Physiology of pain perception has been poorly understood. Recent studies explored the physiology of the perception of acute pain including some that have used brain imaging with electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI). Research supports the idea that perception of pain can be modified by mind-body therapies such as distraction, imagery, and mirror therapy, which are based on the belief that brain processes can strongly influence pain perception.8,18
Modulation
Modulation of afferent input generated in response to noxious stimuli occurs at every level from the periphery to the cortex and involves dozens of neurochemicals.14 For example, serotonin and norepinephrine are central inhibitory neurotransmitters released in the spinal cord and brainstem by descending fibers of the modulatory system (see Fig. 31.1). Some antidepressants provide pain relief by blocking the body’s reuptake of serotonin and norepinephrine, extending their availability to fight pain. Endogenous opioids are located throughout the PNS and CNS, and like therapeutically administered opioids, they inhibit neuronal activity by binding to opioid receptors. As an example, Fig. 31.1 shows that the dorsal horn of the spinal cord, which is densely populated with opioid receptors, is the primary action site of epidural opioids.
Pathophysiology of Neuropathic Pain
Neuropathic pain is sustained by mechanisms driven by damage to, or dysfunction of, the PNS or CNS. In contrast to nociceptive pain, neuropathic pain is abnormal processing of stimuli.12,19 Whereas nociceptive pain involves tissue damage or inflammation, neuropathic pain can occur in the absence of either. Neuropathic pain, even when acute, reflects a pathophysiology that serves no useful purpose.8 A discussion of some of the peripheral and central mechanisms that initiate and maintain neuropathic pain follows. Extensive research is ongoing to better define these mechanisms.
Peripheral Mechanisms
Neuropathic pain can develop at any point from the periphery to the CNS. For example, when nociceptors are injured, changes in the numbers and location of ion channels, particularly sodium channels, can abnormally accumulate.6 The threshold for nerve depolarization is then lowered, which leads to an increased response to stimuli and ectopic discharges. Hyperexcitable nerve endings in the periphery can become damaged, leading to abnormal reorganization of the nervous system, an underlying mechanism of some neuropathic pain states.12 Chemically mediated connections can form between nerve fibers and cause abnormal activation of neurons and ultimately pain. These processes lead to a phenomenon called peripheral sensitization, which is thought to contribute to the maintenance of neuropathic pain. Topical local anesthetics, such as lidocaine patch 5%, are an example of analgesics that produce effects in the tissues under the site of application by “dampening” neuropathic pain mechanisms in the PNS.20
Central Mechanisms
Central mechanisms also have a role in establishing neuropathic pain. Central sensitization is a complex process that is still being studied to improve understanding. It is defined as pain hypersensitivity elicited by abnormal hyperexcitability of central neurons as a result of complex changes induced by incoming barrages of nociceptors.8,21 The accumulation of intracellular ions causes spinal neurons to become highly sensitized and fire rapidly in a process called wind-up that occurs early in the process.9,15 Extensive release and binding of excitatory neurotransmitters, such as glutamate, activate the NMDA receptor and cause an increase in intracellular calcium levels into the neuron resulting in pain. As noted, the NMDA antagonist ketamine directly antagonizes this activity. An increase in the influx of sodium is thought to lower the threshold for nerve activation, increase response to stimuli, and enlarge the receptive field served by the affected neuron.9 Local anesthetics and anticonvulsants can block ion channels and inhibit abnormal pain sensation.
As with injured peripheral neurons, synaptic reorganization and anatomic changes can also occur in the CNS. These are thought to be sustained by an increased responsiveness of central neurons to relatively mild peripheral stimuli.19 For example, injury to a nerve route can lead to reorganization in the dorsal horn of the spinal cord. Nerve fibers can invade other areas and create abnormal sensations in the area of the body served by the injured nerve. Allodynia or pain from a normally nonnoxious stimulus (e.g., touch) is one such abnormal sensation and a common feature of neuropathic pain. In patients with allodynia, the mere weight of clothing or bed sheets can be excruciatingly painful. The ability of the nervous system to change structure and function as a result of noxious stimuli is called neuroplasticity.8
Another underlying mechanism called central disinhibition occurs when control mechanisms along inhibitory (modulatory) pathways are lost or suppressed, leading to abnormal excitability of central neurons.8 Possible causes of disinhibition include dysfunction of the gamma-aminobutyric acid (GABA) pathways. GABA is the most abundant neurotransmitter in the CNS and composes a major inhibitory neurotransmitter system. Increased GABA function may help to relieve neuropathic pain. Benzodiazepines, such as midazolam, enhance GABA function, resulting in analgesia for pathologic conditions like muscle spasm.8,20
Harmful Effects of Unrelieved Pain
Literally every system in the body is affected by unrelieved pain; the harmful effects are numerous (Table 31.2). Unrelieved pain triggers and prolongs the stress response, causing the release of excessive amounts of hormones such as cortisol, catecholamines, and glucagon; insulin and testosterone levels decrease.22,23 This increased endocrine activity initiates a number of metabolic processes that can result in weight loss, tachycardia, increased respiratory rate, shock, and even death.22 Persistent unrelieved pain has been linked to increased tumor growth24 and a higher incidence of health care–associated infections.25
Effects on the cardiovascular (CV) system include increased postoperative blood loss26 and hypercoagulation,22 which can lead to myocardial infarction and stroke. The respiratory system is affected by small tidal volumes and decreases in functional lung capacity, which can lead to pneumonia, atelectasis, and an increased need for mechanical ventilation.27,28
Every surgical procedure has the potential to produce persistent (chronic) postsurgical pain. Although it is not possible to predict which patient will develop it, inguinal hernia repair, amputation, and thoracic, cardiac, and breast surgery are among those identified as high risk for this complication.8,29–31 Multiple factors are thought to contribute to the development of persistent postsurgical pain, including nerve injury from the surgical procedure, preexisting pain, psychosocial factors, genetic susceptibility, and severe postoperative pain.32–34 Persistent postsurgical pain may have nociceptive, inflammatory, and neuropathic components, indicating a need for a multimodal treatment approach.30 Similar to other complex pain syndromes, it can be difficult to treat and last a lifetime.
Pain Assessment: the Foundation
The gold standard for assessing the existence and intensity of pain is the patient’s self-report.2 A comprehensive pain assessment provides the foundation for good pain control and includes obtaining the following information from the patient:
• Location of pain: Ask the patient to state or point to the areas of pain on the body.
• Intensity: Ask the patient to rate the intensity of the pain using a reliable and valid pain assessment tool. A number of scales in several language translations have been evaluated and made available for use in clinical practice and for educational practice.2 See Box 31.1 for practical tips on using self-report pain-rating tools. The most common tools are:
Table 31.2
Harmful Effects of Unrelieved Pain
| Domains Affected | Specific Responses to Pain |
| Endocrine | ↑ ACTH, ↑ cortisol, ↑ ADH, ↑ epinephrine, ↑ norepinephrine, ↑ GH, ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ aldosterone, ↑ glucagon, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac workload, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioral and physiologic responses to pain, altered temperaments, higher somatization, infant distress behavior, possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behavior, and anxiety states |
| Future pain | Debilitating chronic pain syndromes: postmastectomy pain, postthoracotomy pain, phantom pain, postherpetic neuralgia |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
• Numerical Rating Scale (NRS) is most often presented as a horizontal 0- to 10- point scale with word anchors of “no pain” at one end of the scale, “moderate pain” in the middle of the scale, and “worst possible pain” at the end of the scale.
• Wong-Baker FACES Pain Rating Scale consists of six cartoon faces with word descriptors, ranging from a smiling face for “no pain (or hurt)” to a frowning tearful face for “worst pain (or hurt).” The faces are most commonly numbered using a 0, 2, 4, 6, 8, 10 metric; however, 0 to 5 can also be used. Patients are asked to choose the face that best describes their pain. The FACES scale is used in children as young as 3 years old.2 Fig. 31.3 provides the Wong-Baker FACES scale combined with the NRS.
• FACES Pain Scale-Revised (FPS-R) has seven faces to make it consistent with other scales using the 0-to-10 metric. The faces range from a neutral facial expression to one of intense pain and are numbered 0, 2, 4, 6, 8, and 10. Patients are asked to choose the face that best describes their pain. It is important for clinicians to understand that the scale is reliable and valid in children as young as 3 years old, but research in children less than 5 is scarce.35 The FPS-R has been shown to be preferred by both cognitively intact and impaired elders36,37 and minority populations.36,38
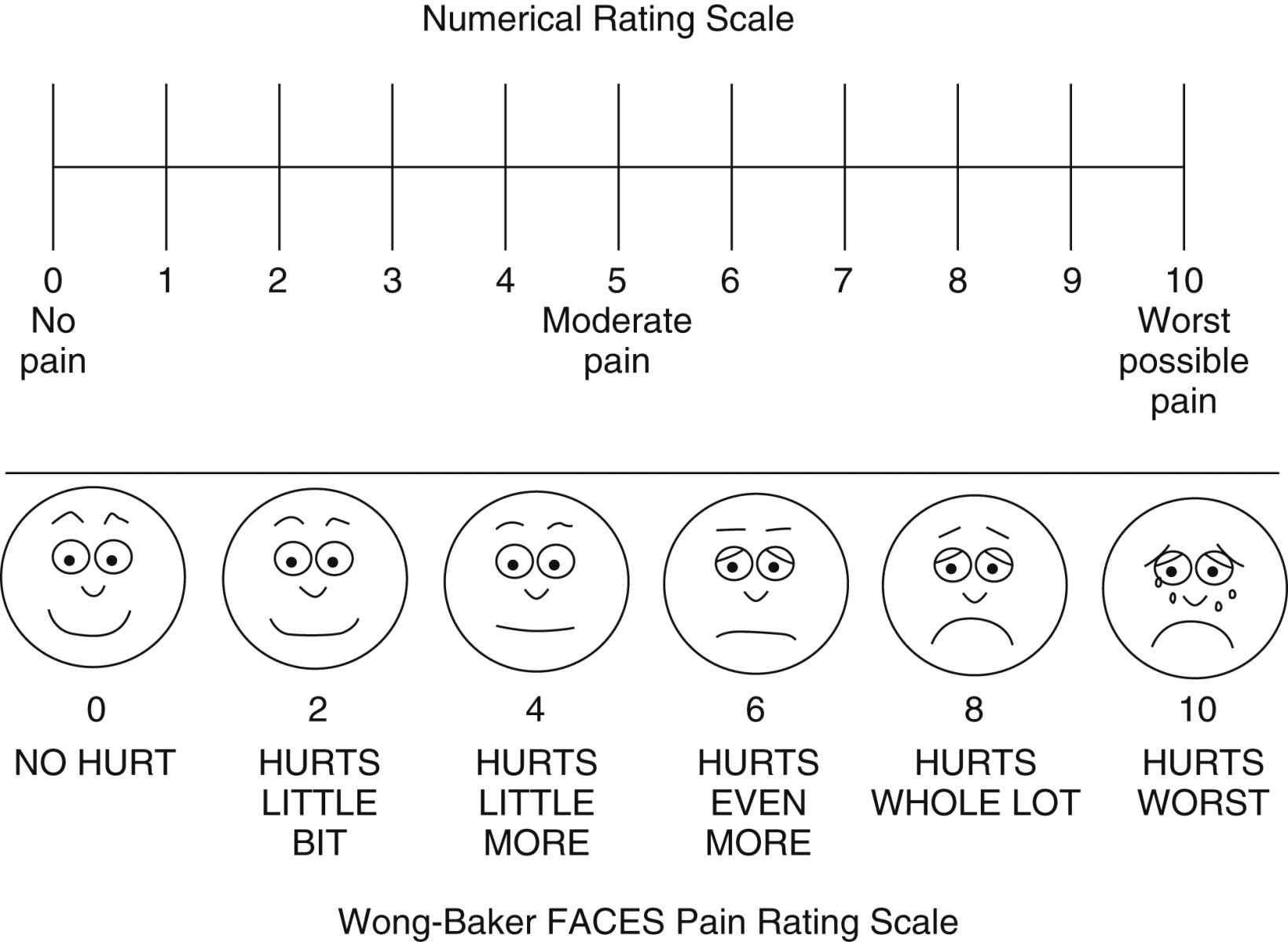
FIG. 31.3 Pain rating scales. A numeric rating scale or visual analog scale is useful for evaluation of adult pain status. The Wong-Baker FACES pain rating scale is useful in rating of pain in the pediatric population. (From Hockenberry MJ, Wilson D: Wong’s nursing care of infants and children, ed 10, St. Louis, MO, 2015, Elsevier. Used with permission.)
• Verbal Descriptor Scales (VDS) use different words or phrases to describe the intensity of pain such as no pain, mild pain, moderate pain, severe pain, very severe pain, and worst possible pain. The patient is asked to select the phrase that best describes the pain intensity. The scale can be presented horizontally or vertically and can be helpful for patients who have difficulty using a numeric scale.2
• Quality: Ask the patient to describe how the pain feels. Descriptors such as sharp, shooting, or burning may help to identify the presence of neuropathic pain.
• Onset and duration: Ask when the pain started and whether it is constant or intermittent.
• Aggravating and relieving factors: Ask the patient what makes the pain worse and what makes it better.
• Effect of pain on function and quality of life: It is particularly important to ask patients with persistent pain how pain affects their lives; what could they do before the pain began that they can no longer do, or what do they want to do but cannot do because of pain?
• Comfort-function (pain) goal: For patients with acute pain, identify short-term functional goals, and reinforce the link between good pain control and successful achievement of the goals. For example, surgical patients are told that they will be expected to cough, deep breathe, turn, and ambulate or participate in physical therapy postoperatively. Patients with chronic pain can be asked to identify their unique functional or quality-of-life goals such as being able to work, walk the dog, or garden. Patients are then asked to identify (using a 0-to-10 scale) a level of pain that is realistic and will allow accomplishment of identified functional or quality-of-life goals with reasonable ease. Pain intensity ratings consistently above the goal warrant further evaluation and consideration of an intervention and possible adjustment of the treatment plan.2
• Other information: The patient’s culture, past pain experiences, and pertinent medical history such as comorbidities, laboratory tests, and diagnostic studies are considered when establishing a treatment plan.39
Challenges in Assessment
Many patients are unable to provide a report of their pain using the customary self-report pain rating tools, placing them at higher risk for undertreated pain than those who can report.2 These patients are collectively called nonverbal patients40 and include infants, toddlers, and patients who are cognitively impaired, critically ill (intubated, unresponsive), comatose, imminently dying, receiving neuromuscular blocking agents, or sedated from anesthesia and other medications given during surgery.
When patients are unable to report pain using traditional methods, an alternative approach based on the Hierarchy of Pain Measures is recommended.2,40,41 The key components of the hierarchy are to (1) attempt to obtain self-report, (2) consider underlying pathology or conditions and procedures that might be painful (e.g., surgery), (3) observe behaviors, (4) evaluate physiologic indicators, and (5) conduct an analgesic trial. See Box 31.2 for detailed information on each component of the Hierarchy of Pain Measures.
Self-report is at the top of the hierarchy and should be attempted even in patients who present challenges in assessment.2 Many patients with mild to moderate cognitive impairment can provide self-report when clinicians implement fairly simple measures (see Box 31.1).
Several behavioral pain assessment tools exist to facilitate assessment in nonverbal patients; however, patients must be carefully evaluated for their ability to respond with the requisite behaviors in the selected tool.2 For example, tools that require assessment of body movement as a pain indicator should not be used in patients who are unable to move such as those receiving a neuromuscular blocking agent. According to the hierarchy, pain can be assumed to be present in these patients, justified by research showing that endotracheal intubation, ventilation, and suctioning—all required in patients receiving a neuromuscular blocking agent—are painful.42,43 It is equally important to understand that the score obtained from the use of a behavioral pain assessment tool helps to identify the presence of pain, but the score is a behavioral score and not a pain intensity rating. Simply put, if the patient cannot report the intensity of the pain, the intensity is not known.2,44 It is also important to remember that the absence of behavior does not mean the absence of pain.
Although nurses who care for patients with acute pain often rely on vital signs to assess pain, these physiologic signs are considered poor indicators of pain.2,45,46 Many factors other than pain can influence changes in vital signs, and patients quickly adapt physiologically despite the presence of pain. The primary message is that the absence of an elevated blood pressure or heart rate does not mean the absence of pain.2,35
Reassessment of Pain
After initiation of the pain management plan, pain is reassessed and documented on a regular basis as a way to evaluate the effectiveness of treatment. At a minimum, pain should be reassessed with each new report of pain and before and after administration of analgesics.2 The frequency of reassessment depends on the stability of the patient’s pain and the pharmacokinetics and pharmacodynamics of the medication and is guided by institutional policy. For example, in the postanesthesia care unit (PACU), reassessment may be necessary as often as every 10 minutes when pain is unstable during opioid titration but can be done every 4 to 8 hours in patients with stable pain 24 hours after surgery. It is strongly recommended that sedation and respiratory status is reassessed with each reassessment of pain.
Pain Control on a Continuum
The quality of patients’ pain control should be addressed when patients are discharged from one clinical area to another. Many PACUs establish the criterion that patients must achieve a pain rating of 4 on a scale of 0 to 10 or better before discharge; however, the expectation that all patients must be discharged from a given clinical unit with pain ratings less than an arbitrary number is unrealistic. This can lead to the unsafe administration of additional opioid doses to patients who are excessively sedated and is widely discouraged.7,39,47–49 Instead, achieving optimal pain relief is best viewed on a continuum with the primary objective being to provide both effective and safe analgesia.7,39,49 Optimal pain relief is the responsibility of every member of the health care team and begins with analgesic titration in the PACU followed by continued prompt assessment and analgesic administration after discharge from the PACU to achieve pain ratings allowing patients to meet their functional goals with relative ease.
Although it may not always be possible to achieve a patient’s pain rating goal within the short time the patient is in an area like the PACU, this goal provides direction for ongoing analgesic care. Important information to give to the nurse assuming care of the patient on the clinical unit is the patient’s pain rating goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics and doses), and how well the patient has tolerated analgesic administration (adverse effects).39
Multimodal Analgesia: Pharmacologic Management of Pain
Pain is a complex phenomenon involving multiple underlying mechanisms. This characteristic underscores the importance of using more than one analgesic to manage pain.39 This approach is called multimodal analgesia. It involves using pharmacologic and nonpharmacologic interventions and is recommended for the treatment of all types of pain.4,28,50 A multimodal regimen combines medications with different underlying mechanisms; this allows lower doses of each medication in the treatment plan, which reduces the potential for each to produce adverse effects.39 Furthermore, multimodal analgesia can result in comparable or greater pain relief than can be achieved with any single analgesic. Multimodal analgesia should be the rule rather than the exception in pain treatment.
The most common analgesics used for postoperative pain management are nonopioid analgesics (e.g., acetaminophen, NSAIDs), opioid analgesics (e.g., morphine, hydromorphone, fentanyl, and oxycodone), local anesthetics, and anticonvulsants. A multimodal approach in the perioperative setting may combine agents from each of these analgesic groups to provide effective pain relief and help minimize adverse effects. Unless contraindicated, all surgical patients should routinely be given acetaminophen and an NSAID in scheduled doses throughout the postoperative course. It is preferable that these medications are initiated preoperatively with the goal to reduce surgical pain.51 Opioid analgesics are added to manage moderate to severe postoperative pain in most patients. For some major surgical procedures, a local anesthetic is administered with an opioid epidurally or alone by continuous peripheral nerve block. An anticonvulsant may be added to the treatment plan as well to control severe pain or prevent a chronic postsurgical pain syndrome such as persistent pain after thoracotomy or mastectomy.8 A metaanalysis found alpha-2 agonists used postoperatively resulted in less pain intensity, less morphine used, and less nausea, yet hypotension and bradycardia were concerning side effects.52
Routes of Administration
One principle of pain management is to use the oral route of administration whenever feasible.39 When the oral route is not possible (e.g., patients who cannot swallow, can receive nothing by mouth, or are nauseated), other routes of administration are used. In the perioperative setting, the intravenous (IV) route is the first-line route of administration for analgesic delivery; patients are transitioned postoperatively to the oral route as tolerated. Other methods to manage pain use catheter techniques, such as intraspinal analgesia and continuous peripheral nerve block infusions. Nurses have an extensive role in the successful management of these therapies. The American Society for Pain Management Nursing (http://www.aspmn.org) provides guidelines for care.3
Topical local anesthetics are used for acute procedural pain. Other second-line routes of administration, such as transdermal and subcutaneous, are generally reserved for management of chronic pain. The primary disadvantages of transdermal medication delivery are that the skin serves as both a barrier and a reservoir. There is significant lag time before the effects of the medication are felt after transdermal patch application, and the medication continues to enter the systemic circulation for a variable period after the patch is removed.53
Nonopioid Analgesics
The nonopioid analgesic group includes acetaminophen and NSAIDs. There are two categories of NSAIDs: the nonselective NSAIDs (e.g., ibuprofen, naproxen, diclofenac, ketorolac), which inhibit both COX-1 and COX-2, and the COX-2 selective NSAIDs (e.g., celecoxib), which inhibit only COX-2 (see Fig. 31.2).
Nonopioids are flexible analgesics used for a wide spectrum of painful conditions. They are appropriate alone for mild to some moderate nociceptive-type pain (e.g., from surgery or trauma) and are added to opioids, local anesthetics, or anticonvulsants as part of a multimodal analgesic regimen for more severe nociceptive pain.39,54 Acetaminophen and an NSAID can be given concomitantly, and there is no need for staggered doses.54,55
Acetaminophen is versatile in that it can be given by multiple routes of administration including oral, rectal, and IV. IV acetaminophen is approved for treatment of pain and fever in adults and children 2 years of age and older and is given by a 15-minute infusion in single or repeated doses. It can be given alone for mild to moderate pain or moderate to severe pain with adjunctive opioid analgesics and has been shown to be well tolerated and to produce a significant opioid dose-sparing effect and superior pain relief when compared with placebo.56 Postoperative nausea and vomiting (PONV) have been reduced with IV acetaminophen.57 The maximum daily dose for IV acetaminophen is the same as for oral acetaminophen (e.g., 1000 mg every 6 hours, for maximum of 4000 mg in adults and adolescents weighing more than 50 kg; 15 mg/kg every 6 hours in adults, adolescents, and children weighing less than 50 kg).
For surgical pain, an NSAID can be added to both acetaminophen and an opioid as part of a multimodal plan, with the combination most often resulting in improved analgesia with fewer side effects and less opioid consumption.58 A metaanalysis of perioperative NSAID use in children reported that opioid use and PONV were lessened.59 This is likely related to the opioid sparing effects of NSAIDs.58
Ketorolac and ibuprofen are available in IV formulation. An abundance of research has shown ketorolac to be effective for postoperative pain following a wide variety of surgical procedures.54,60 Although further clinical experience and research are needed with IV ibuprofen, it is less COX-1 selective than ketorolac,54 which may result in fewer adverse effects than ketorolac (see Fig. 31.2). A multicenter study (n = 300) investigated patients with various types of surgeries. When the majority of patients (84%) were given a single preoperative dose of IV ibuprofen, they reported reduced pain and used more than 30% less opioid. The remaining patients were given two or more doses. The most common adverse event was infusion site pain.61
Adverse Effects of Nonopioids
Acetaminophen is widely considered one of the safest and best tolerated analgesics.62,63 Its most serious complication is hepatotoxicity (liver damage) as a result of overdose. In the healthy adult, a maximum daily dose under 4000 mg is rarely associated with liver toxicity.64 Concerns about accidental overdose of IV acetaminophen in children are related to errors in inaccurate dosing.65 Because in first-pass models there was 50% less acetaminophen exposure to the liver with IV acetaminophen when compared with oral, there may be less risk with the IV route.66 Its lack of effect on platelet aggregation and low incidence of gastrointestinal (GI) adverse effects make acetaminophen the analgesic of choice in individuals with renal insufficiency, advanced chronic kidney disease, and end-stage renal disease.67,68 Acetaminophen has been shown to increase the International Normalized Ratio when administered with warfarin, but the likelihood of surgical bleeding as a result of perioperative acetaminophen intake is thought to be low.69,70
The NSAIDs reportedly have significantly more adverse effects than acetaminophen including bleeding, oliguria, renal dysfunction, and gastric toxicity/ulceration.71–74 However, two recent metaanalyses reported NSAIDs to be safe without increased risk of bleeding.75,76 The primary underlying mechanism of NSAID-induced gastric ulceration is the inhibition of COX-1, which leads to a reduction in GI-protective prostaglandins (see Fig. 31.2).62 This effect is systemic rather than local and can occur regardless of the route of administration of the NSAID.54 GI adverse effects are also related to the dose and duration of NSAID therapy; the higher the NSAID dose and the longer the duration of NSAID use, the higher the risk of cumulative GI toxicity.74 This fact underscores the importance of administering the lowest dose for the shortest time necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree