Innervations
Sympathetic
Parasympathetic
Afferent sensory fibers
Kidney
T8–L1 preganglionic fibers
Coming from the vagus nerves
Spinal root ganglia T10-1112
Ureter: upper part
Celiac and aorticorenal ganglia
Nerve fibers transverse the celiac plexus
Transverse paravertebral sympathetic
Lesser and least splanchnic nerves
Ganglia
Ureter: lower part
L1–L2 preganglionic fibers
Coming from S2 to S4
Dorsal spinal root gangliaT12 and L1
Paravertebral sympathetic chain
Nervi erigentes
Superior hypogastric plexus
Inferior hypogastric plexus
Urinary bladder
T12–1–L2 preganglionic fibers
S2–S4
T11–L1
Paravertebral sympathetic ganglia
Nervi erigentes
Accompany both the sympathetic and parasympathetic efferent pathways
Hypogastric plexus
Nervus pudendus
Urethra Male: prostatic and cavernous plexuses (contain sympathetic, parasympathetic, and afferent sensory fibers) – nervus pudendus
Female: vaginal plexus (contain sympathetic, parasympathetic, and afferent sensory fibers) – nervus pudendus
Male genitalia (testes)
Renal plexus and intermesenteric nerve fibers in the region from T12 to L2
No somatic innervation
Prostate gland
Prostatic plexus contain sympathetic and parasympathetic nerve fibers
Spinal root ganglia T10
Scrotum
Nervus ilioinguinalis
Genital branch of the nervus genitofemoralis (L1–L2)
Nervus pudendus
Penis
From the sacral spinal cord (S2–S4) via the hypogastric plexus
Branches from the pudendal nerve (S2–S4)
Kidney and Ureter
The only sensation that can be evoked from the ureter is pain, whereas other organs such as the bladder can give rise to several sensations ranging from mild fullness to pain. Several groups of ureteric sensory afferents can be distinguished and triggered by contraction and dilatation of the ureter. Pain coming from the kidney is often localized in the region of the costovertebral angle. Distention of the upper part of the ureter causes pain adjacent to the anterior superior iliac spine. Pain coming from the distal part is localized in the suprapubic area. Pain due to a ureteral stone is often referred to the ipsilateral groin, scrotal or labial skin, or medial thigh. Hyperalgesia may be present in the T10 to L1 dermatomes. Understanding of the ureteral function and physiology is mandatory for developing new drugs, for example, in renal colic (Fig. 14.1).
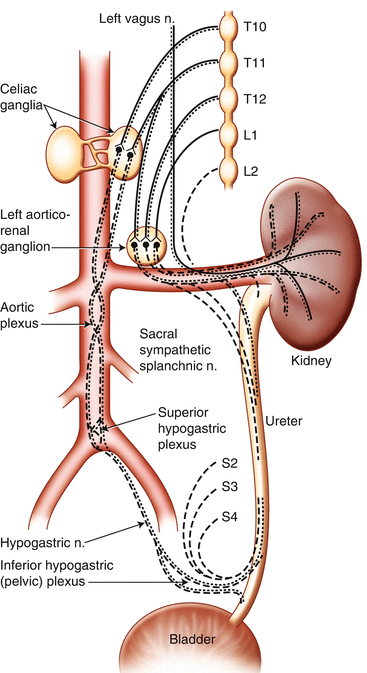

Fig. 14.1
Urogenital innervation
Urinary Bladder
Two distinct groups of sensory afferent fibers capable of signaling noxious stimuli have been identified in the urinary bladder. Graded distension of the healthy urinary bladder in humans initially gives rise to a sensation of fullness and eventually pain (in the suprapubic region) as volume increases and intravesical pressure exceeds about 25–35 mmHg [18–20]. In the inflamed bladder, the sensations during bladder emptying become unpleasant and painful. Uninhibited bladder contractions may be interpreted as bladder pain or spasm.
Prostate
Prostate pain may be accompanied by sensations of rectal discomfort, tenesmus, and perineal pain. Disorders of the seminal vesicles (often associated with prostate disease) cause pain in the lateral lower abdominal region or groin with referred pain to the perineum and penis.
Scrotum and Penis
Testicular pain may be experienced in the groin or the lower abdomen. Scrotal pain may mimic renal colic. Pain in the scrotal region may be referred pain coming from the ureter or the bladder. Pain of the penis (skin or corporal bodies) is transmitted directly to the nerve roots of S2–S4.
In summary, pain from the urogenital region may often be difficult to comprehend because referred pain (pain from the bladder is frequently referred to the bladder and patients with flank pain may experience a disorder of the genitalia) is the most common pain experienced in the urogenital system.
Chronic Pelvic Pain Syndromes
The most common definition for chronic pelvic pain is nonmalignant pain perceived in structures related to the pelvis, constant or recurring over a period of more than 6 months [36–43].
Pain perceived within the pelvis may arise from a range of different mechanisms, many of which remain poorly understood. The relationship between pain and an underlying pathology which explain it is not always simple. Many patients referred to a urologist complain about a mixture of symptoms regarding the pelvic area, and the clinician is often confronted with a considerable overlap of pelvic pain, voiding symptoms, sexual dysfunction, and presence of infection.
Sources of pain may be located in the reproductive urinary, or gastrointestinal tract, the central nervous system, or in musculoskeletal structures. Additionally, psychological factors may play a part in the development or maintenance of persistent pelvic pain. Pain may disrupt daily life and cause distress including altered emotional well-being (anxiety, depression), changes in body perception, changes in professional and social function, increased dependency, and distress from retrospection. These issues may have a profound adverse impact on the patient’s quality of life. In this view, adequate treatment of chronic pelvic should target the complex and interwoven relationship between pain, physical deteriorations, and psychosocial distress.
Poor understanding of pathogenesis of chronic pelvic pain and lack of consensus of the definition of chronic pelvic pain and universally accepted diagnostic criteria for some clinical conditions greatly hinder diagnosis and may lead to false interpretation of the symptoms and consecutively to inappropriate therapy. In this view, a classification system has been proposed by the European Association of Urology to guide the clinician through the process from diagnosis to evidence-based management of chronic pelvic pain. A keynote in this classification is to make clear that patients may experience significant pain without finding (matching) pathologic changes.
Chronic pelvic pain syndromes include prostate pain syndrome (chronic prostatitis, prostadynia), bladder pain syndrome (interstitial cystitis), scrotal pain syndrome, urethral pain syndrome, pelvic pain syndromes of gynecological origin (endometriosis, dysmenorrhea, vaginal or vulvar pain syndromes), neurogenic pain conditions (conus or sacral root pathology, pudendal nerve entrapment), pelvic floor function and dysfunction (myofascial trigger points), and pain from anorectal origin (proctitis, anal fissure) (Table 14.2).
Table 14.2
Classification of chronic pelvic pain syndromes (based on the European Association of Urology)
System | End organ as pain syndrome | |
|---|---|---|
Urogenital | Bladder pain syndrome | Further diagnosis based on results from biopsy (cystoscopy) |
Urethral pain syndrome | Urethritis | |
Prostate pain syndrome | Inflammatory | |
Noninflammatory | ||
Scrotal pain syndrome | Testicular pain syndrome | |
Epididymal pain syndrome | ||
Penile pain syndrome | Post-vasectomy pain syndrome | |
Endometriosis associated pain | ||
Gynecology | Vaginal pain syndrome | |
Vulvar pain syndrome | Generalized vulvar pain syndrome | |
Localized vulvar pain syndrome | ||
Vestibular pain syndrome | ||
Clitoral pain syndrome | ||
Anorectal | Proctitis | |
Anal fissure | ||
Hemorrhoids | ||
Neuromuscular | Pudendal neuropathy | |
Sacral spinal cord pathology |
The general principles in the approach to patients with chronic pelvic pain are fairly simple. Validated questionnaires to assess and quantify the symptoms are mandatory. Basic investigations should be performed to rule out well-defined and treatable pathologies. In the absence of abnormal findings and the lack of evidence of infectious, allergic, or oncologic causes, it is unlikely that the patients suffer from a well-defined illness (e.g., acute bacterial prostatitis amenable to antibiotics).
Patients with chronic pelvic pain need a multimodal approach because many factors may contribute to these pain syndromes. Besides physical signs suggesting a pathophysiologic (somatic) mechanism, there is strong evidence for the involvement of cognitive and emotional processes in pain processing. Additionally sexual problems are common in patients with chronic pelvic pain. Chronic pain affects sexual function and sexual dysfunction may heighten anger, frustration, and depression. Furthermore, associations between sexual or physical abuse and chronic pelvic pain in women were found in some studies.
Anxiety, depression, and sexual problems should be addressed in assessment and treatment of chronic pelvic pain. A multidisciplinary approach integrating physical and psychosocial interventions (psychologists and/or sexologists) has to be emphasized.
Guidelines for treatment of chronic pelvic pain are derived from the general chronic pain literature and include pharmacological (the three-step analgesic ladder as proposed by the WHO, neuropathic analgesics) and sacral neuromodulation. Sacral neuromodulation has been shown to benefit patients with refractory motor urge incontinence [16, 17], urinary retention, and chronic pelvic pain syndromes. Electrical stimulation of the S3 and S4 sacral nerves (transforaminal approach) seems to modulate neural reflexes of the pelvis resulting in an improvement in refractory urinary voiding and relief of pelvic pain [36–43].
The Management of Acute Pain
The Undertreatment of Acute Pain
The treatment of acute pain is recognized as an important health-care issue. Despite this recognition and the introduction of standards and guidelines, data from around the world suggest that postoperative pain continues to be under managed [44]. Prevalence of moderate or severe pain at rest was especially high on the day of surgery and on the first postoperative day (30–55%) in the abdominal surgery group. In a random sample of adults who had undergone surgical procedures in the United States, approximately 80% of patients experienced acute pain after surgery. Of these patients, 86% had moderate to extreme pain, with more patients experiencing pain after discharge than before discharge [45]. Again abdominal operations were among the most painful procedures [46].
The Importance of Pain Management
Undertreatment of pain is considered poor medical practice that may result in many adverse effects. Unrelieved pain after surgery can activate efferent sympathetic nerves and increase heart rate, systemic vascular resistance, and circulating catecholamine levels, placing patients at risk of myocardial ischemia, stroke, bleeding, and other complications. Enhanced sympathetic activity can also reduce gastrointestinal (GI) motility and contribute to ileus. Severe pain after upper abdominal and thoracic surgery decreases the ability to cough and reduces functional residual capacity, resulting in atelectasis and ventilation-perfusion abnormalities, hypoxemia, and an increased incidence of pulmonary complications. The injury response also contributes to a suppression of cellular and humoral immune functions and a hypercoagulable state following surgery, both of which can contribute to postoperative complications. Patients at greatest risk of adverse outcomes from unrelieved acute pain include very young or elderly patients, those with concurrent medical illnesses, and those undergoing major surgery [47].
Chronic Postsurgical Pain (CPSP)
Pain that persists after the surgical wound has healed is a major clinical problem. Acute postoperative pain is followed by persistent pain in 10–50% of individuals after common operations, such as groin hernia repair (10%), breast and thoracic surgery (20–40%), and coronary artery bypass surgery (30–50%) [48]. Surgical procedures with the greatest incidence of chronic postsurgical pain are associated with intentional or unintentional nerve damage. The most consistent risk factor for chronic postsurgical pain is the presence and/or intensity of prior pain experienced either preoperatively or early postoperative pain developing in the days and weeks after surgery [49, 50].
Systemic Analgesic Techniques
Paracetamol
Although paracetamol (acetaminophen) is one of the world’s most widely used analgesics, the mechanism by which it produces its analgesic effect is largely unknown. Today it is assumed that paracetamol has a pharmacological mechanism that interacts with a variety of physiological pathways, likely within the central nervous system [51]. Paracetamol as a single agent is an effective analgesic for mild to moderate acute pain. A single dose (1,000 mg of paracetamol) provides effective analgesia for about half of patients with acute postoperative pain, for a period of about 4 h, and is associated with few, mainly mild, adverse events [52]. Paracetamol is also useful in the treatment of moderate to severe pain when combined with other analgesics. Nonselective NSAIDs (non-steroidal anti-inflammatory drugs) given in addition to paracetamol improve analgesia compared to paracetamol alone [53, 54]. Paracetamol is also an effective adjunct to opioid analgesia, opioid requirements being reduced by 20 –30% when combined with a regular regimen of oral or rectal paracetamol [54]. The use of oral paracetamol in higher daily doses (1 g every 4 h) in addition to PCA morphine lowered pain scores, shortened the duration of PCA use, and improved patient satisfaction [55]. Paracetamol has fewer side effects than NSAIDs and can be used when the latter are contraindicated (e.g., patients with a history of asthma or peptic ulcers). It is commonly recommended that paracetamol should be used with caution or in reduced doses in patients with active liver disease, history of heavy alcohol intake, and glucose-6-phosphate dehydrogenase deficiency.
Nonselective NSAIDs and Coxibs
The term NSAIDs is used to refer to both nonselective NSAIDs and coxibs (COX-2 selective inhibitors). NSAIDs have a spectrum of analgesic, anti-inflammatory, and antipyretic effects and are effective analgesics in a variety of acute pain states. All NSAIDs primarily target the synthesis of prostaglandins and are known to be involved in numerous physiological systems such as the regulation of vascular tone, platelet aggregation, protective effects on the gastric mucosa, and regulation of the inflammatory cascade and renal perfusion. Cyclooxygenase (COX) is the enzyme responsible for the synthesis of prostaglandins, thromboxane, and leukotrienes by conversion of arachidonic acid. Cyclooxygenase is known to be present in at least two isomeric forms (COX-1 and COX-2) with different physiological effects. COX-1 is a constitutive enzyme (i.e., “daily household”) and is involved in the production of “physiological” prostaglandins. COX-2 is classically described as inducible and is expressed in inflamed/traumatized tissues but is lacking in others (e.g., platelets) [56].
Single doses of nonselective NSAIDs are effective in the treatment of moderate to severe pain after surgery, renal colic [57], and primary dysmenorrhea. Nonselective NSAIDs are integral components of multimodal and preventive analgesia [58]. However, while useful analgesic adjuncts, they are inadequate as the sole analgesic agent in the treatment of severe postoperative pain. When given in combination with opioids after surgery, nonselective NSAIDs resulted in better analgesia, reduced opioid consumption, and a lower incidence of postoperative nausea and vomiting (PONV) and sedation [59]. There was no effect on pruritus, urinary retention, and respiratory depression [60]. The combination of nonselective NSAIDs and paracetamol is effective [53]. Current evidence suggests that a combination of paracetamol and an NSAID may offer superior analgesia compared with either drug alone [61]. Coxibs are as effective as nonselective NSAIDs in the management of postoperative pain [62]. Preoperative coxibs reduced postoperative pain and opioid consumption and increased patient satisfaction [63].
Adverse Effects
Although very effective, adverse effects of nonselective NSAIDs are significant and may limit their use. In the perioperative period the main concerns are renal impairment, interference with platelet function, wound and bone healing, and peptic ulceration or bronchospasm in individuals at risk. In general, the risk and severity of nonselective NSAID-associated side effects are increased in elderly people [64]. Although the adverse renal effects of chronic nonselective NSAIDs and coxibs are common and well recognized, NSAIDs only cause a clinically unimportant transient reduction in renal function in the early postoperative period in patients with normal preoperative renal function. The risk of adverse renal effects of nonselective NSAIDs and coxibs, however, is increased in the presence of factors such as preexisting renal impairment, hypovolemia, hypotension, and the use of other nephrotoxic agents and angiotensin-converting enzyme (ACE) inhibitors [65].
Nonselective NSAIDs inhibit platelet function but the clinical effect seems to be limited. No surgical bleeding complications were reported with COX-2 inhibitors. Coxibs do not impair platelet function because platelets produce only COX-1, not COX-2 [66]. Nonselective NSAIDs cause gastrointestinal side effects, ranging in severity from mild dyspepsia to gastric hemorrhage and perforation, potentially resulting in admission to hospital, surgery, and even death. Use of ketorolac and piroxicam carried the highest risk. The risk is increased with higher doses, a history of peptic ulceration, and use for more than 5 days and in elderly people [67]. Concurrent use of a proton pump inhibitor (PPI) significantly reduced the incidence of nonselective NSAID-related peptic ulcer disease [68]. GI complications are less likely with the use of coxibs compared with nonselective NSAIDs; the incidence was lowest with celecoxib. The best gastroprotective strategy was the combination of a coxib and a PPI.
Information relating to the cardiovascular risks associated with the use of nonselective NSAIDs and coxibs is derived from long-term treatment data and may not reflect the risk of short-term use in the acute pain setting. The FDA concluded that “Short-term use of NSAIDs to relieve acute pain, particularly at low doses, does not appear to confer an increased risk of serious adverse cardiovascular events.” Cardiovascular risk needs to be taken into account when prescribing any non-steroidal anti-inflammatory drugs [69].
Opioids
Opioids are the mainstay of systemic analgesia for the treatment of moderate to severe acute pain. They form an essential part of a multimodal analgesia regimen. Although several new synthetic strong opioids have emerged in the past century, morphine is still the most widely used opioid throughout the world. The key principle for safe and effective use is to titrate the dose against pain relief and to minimize unwanted side effects [70]. Opioids can be administered orally, intravenously, subcutaneously, transdermally, epidurally, intrathecally, and intramuscularly. The intramuscular route however has lost favor and is less commonly used due to the ready availability of intravenous (IV) medications and the unnecessary pain and erratic absorption associated with this specific delivery method [71, 72]. Patients may control postoperative pain by self-administration of intravenous opioids using devices designed for this purpose (patient-controlled analgesia: PCA). PCA is an efficacious alternative to conventional systemic analgesia for postoperative pain control. PCA provides better pain control and greater patient satisfaction than conventional parenteral “as-needed” analgesia. Patients using PCA consumed higher amounts of opioids than the controls and had a higher incidence of pruritus (itching) but had a similar incidence of other adverse effects. Most experience exists with the use of morphine though all full opioid agonists given in appropriate doses produce the same analgesic effect and therapeutic index. Several opioids can be used in patient-controlled analgesia (Table 14.3).
Table 14.3
Typical PCA dosing schedule
Drug (concentration) | Bolus size | Lockout interval (min) | Continuous infusion |
|---|---|---|---|
Morphine (1 mg/mL) | 0.5–2.5 mg | 5–10 | 0.01–0.03 mg/kg/h |
Fentanyl (0.01 mg/mL) | 10–20 μg | 5–10 | 0.5–0.1 μg/kg/h |
Pethidine (10 mg/mL) | 5–25 mg | 5–10 | – |
Codeine
Codeine is classified as a weak mu-opioid receptor agonist. Its analgesic action depends on the metabolism of about 10% of the dose given to morphine by the enzyme CYP2D6. In Caucasian populations, 8–10% of people are poor metabolizers; however 3–5% are ultrarapid metabolizers [73, 74]. Those who are ultrarapid metabolizers have significantly higher levels of morphine and morphine metabolites after the same dose of codeine [75].
Tramadol
Tramadol is commonly referred to as an atypical, centrally acting analgesic because of its combined effects as an opioid agonist and a serotonin and noradrenalin reuptake inhibitor [74]. Although an effective analgesic, it may not provide adequate pain relief if used as the sole agent for the management of moderate to severe acute pain [76]. Tramadol was found to be effective in the treatment of neuropathic pain. The combination of tramadol with morphine is infra-additive and therefore should be discouraged [77]. Tramadol is metabolized by CYP2D6 (as is codeine) and the resultant active metabolite, O-desmethyltramadol, is a more potent mu-opioid receptor agonist than the parent drug [74]. Patients who are poor metabolizers may get less analgesic effect from tramadol. Tramadol’s adverse effect profile differs from other opioids. The risk of respiratory depression is significantly lower at equianalgesic doses, and it does not depress the hypoxic ventilatory response [78]. Significant respiratory depression has only been described in patients with severe renal failure, most likely due to accumulation of the metabolite O-desmethyltramadol [79]. In addition, tramadol has less effect on gastrointestinal motor function than morphine [80]. Nausea and vomiting are the most common adverse effects and occur at rates similar to other opioids.
Morphine
Morphine remains the most widely used opioid for the management of pain and the standard against which other opioids are compared. Morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), the main metabolites of morphine, are formed by morphine glucuronidation, primarily in the liver [81]. M6G is a mu-opioid receptor agonist that crosses the blood-brain barrier more slowly than morphine, contributes to morphine analgesia in patients with both normal and impaired renal function, and has other morphine-like effects including respiratory depression; M3G has very low affinity for opioid receptors, has no analgesic activity, and animal studies have shown that it may be responsible for the neurotoxic symptoms (not mediated via opioid receptors), such as hyperalgesia, allodynia, and myoclonus, sometimes associated with high doses of morphine [82]. Both M6G and M3G depend on renal excretion. Impaired renal function, the oral route of administration (first pass metabolism), higher doses, and increased patient age are predictors of higher M3G and M6G concentrations [83] with the potential risk of severe long-lasting sedation and respiratory depression.
Fentanyl
Fentanyl is a highly potent phenylpiperidine derivative, structurally related to pethidine (Demerol). It is metabolized almost exclusively in the liver to minimally active metabolites. Less than 10% of unmetabolized fentanyl is renally excreted. Fentanyl is commonly used in the treatment of acute pain, especially when its lack of active metabolites and fast onset of action may be of clinical benefit [84].
Buprenorphine
Buprenorphine is a synthetic partial mu-opioid receptor agonist and kappa-opioid receptor antagonist with high receptor affinity and slow dissociation from the mu-receptor. Mean terminal half-lives are 24 h following sublingual administration and 2–3 h after parenteral injection; two-thirds of the drug is excreted unchanged, mainly in feces, while the remaining one-third is metabolized predominantly in the liver and gut wall via glucuronidation to an inactive metabolite, buprenorphine-3-glucuronide, and via CYP3A4 to norbuprenorphine, which has 40 times less analgesic effect than buprenorphine [84]. Maximum onset of effect is slower than for other opioids making acute titration difficult. In clinically relevant doses, buprenorphine appears to behave like a full mu-opioid receptor agonist, and in animals as well as humans in low doses (i.e., transdermal buprenorphine), there also appears to be no antagonism of other concurrently administered mu-agonist drugs. Contrary to earlier concerns, there was a ceiling effect found for respiratory depression but not for analgesia [81]. The risk of respiratory depression is low compared with morphine, methadone, hydromorphone, and fentanyl, even in the doses used for the treatment of opioid addiction, as long as concurrent sedative medications are not given [84]. Should buprenorphine-induced respiratory depression occur, reversal is possible although higher-than-usual doses and a longer duration infusion of naloxone may be required [81].
Patient Age
Older surgical patients differ from younger patients in many ways, including physical status, medication use and previous pain experiences. Age-related patterns in pain and opioid requirements are multi-determined and the same factor may have different effects across age groups [85]. Age is known to be a better clinical predictor of postoperative opioid requirement than patient weight, with an inverse relationship between average dose and age [85]. The decrease in opioid requirement is not associated with reports of increased pain. This age-related decrease in opioid requirement is due mainly to differences in pharmacodynamics or brain penetration rather than systemic pharmacokinetic factors [86]. Initial use of lower doses in older patients is suggested, but doses should be increased, in the absence of side effects, if analgesia is inadequate [87].
Adverse Effects of Opioids
Common adverse effects of opioids are respiratory depression, sedation, pruritus, nausea, vomiting, slowing of gastrointestinal function, and urinary retention. Combining paracetamol or NSAIDs with PCA morphine induced a significant morphine-sparing effect [59].
Respiratory Depression
Respiratory depression (defined as decreased central CO2 responsiveness resulting in hypoventilation and elevated PaCO2 levels) is thought to be the most important adverse effect when considering analgesic techniques. Considerable variability between studies in the criteria used for defining respiratory depression is reported. A review on respiratory depression after intramuscular analgesia, patient-controlled analgesia (PCA), and epidural analgesia after major surgery reported an overall mean incidence of respiratory depression of the three analgesic techniques of 0.3% using requirement for naloxone as an indicator. In a meta-analysis comparing PCA versus conventional analgesia, 19% of patients in the PCA group versus 21% of those in the control group reported sedation. No difference in the incidence of sedation between the groups was found [87]. A respiratory depression is almost always preceded by sedation, the best early clinical indicator is increasing sedation although monitoring respiratory rate is still important.
Nausea and Vomiting
PONV is common and related to opioid administration in a dose-dependent manner, although many other risk factors for PONV have also been identified [88]. Drugs used as components of multimodal analgesia and which are opioid sparing may reduce PONV. Opioid sparing and a reduction in PONV have been shown with concurrent administration of gabapentin [89], nonselective non-steroidal anti-inflammatory drugs [90], and ketamine.
Impairment of Gastrointestinal Motility
Opioids impair return of bowel function after surgery. Combining peripheral acting opioid antagonists with opioids seem promising in preventing this side effect. A meta-analysis in patients receiving opioids for various reasons including postoperative pain demonstrated that methylnaltrexone and alvimopan are efficacious in reversing opioid-induced increased gastrointestinal transit time and constipation, and that alvimopan is safe and efficacious in treating postoperative ileus. Avoiding the use of systemic opioids by regional anesthesia also reduces bowel dysfunction [90].
Regional Analgesic Techniques
Epidural analgesia provides excellent postoperative pain relief for extended periods after major surgical operations, reducing postoperative complications and the consumption of opioids. Additionally, patient-controlled epidural analgesia allows individualization of dosage, decrease in the use of drugs, and greater patient satisfaction. It also seems to provide better analgesia than intravenous PCA.
Other techniques such as local anesthetic blocks (intermittent and continuous infiltration of the ilioinguinal or iliohypogastric nerves) and intraoperative wound infiltration followed by continuous postoperative wound instillation using a multi-hole catheter can be used after urological surgical operations to supplement postoperative analgesia reducing consumption of systemic analgesics [91–93].
Multimodal Analgesia
The necessary use of analgesics comes with side effects, especially when agents are used in higher dosage. Combining agents with different mechanisms of action may have synergistic effects in preventing or treating acute pain, while lower doses of drugs reduce side effects [94]. A strategy that uses more than one class of analgesic agent is called multimodal analgesia. This technique has been advocated as a means to improve analgesia through either additive or synergistic effects while reducing opioid-related side effects. Multimodal analgesia realistically can be defined as a combination of an opioid and non-opioid analgesic, with or without a regional anesthetic block, typically resulting in improved analgesia with concurrent reduction in the incidence of some opioid-related side effects (e.g., a decrease in postoperative nausea, vomiting, and sedation), presumably through an opioid sparing effect [95]. Perioperative, multimodal preventive analgesia regimens have been shown to protect against the development of chronic postsurgical pain and the incidence and/or the intensity of chronic postsurgical pain.
Pathophysiology of Neuropathic Pain
Neuropathic pain is defined by the IASP as “pain initiated or caused by a primary lesion or dysfunction of the nervous system” [96]. While this definition has been useful in distinguishing some characteristics of neuropathic and nociceptive types of pain, a more precise definition has been developed (reference): pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.
Trauma to neural tissue produces abnormalities of neural function that are perceived by the patient as the symptoms and signs of neuropathic pain. On examination, both negative and positive sensory symptoms may be present. Positive signs include pain, paresthesia, dysesthesia, hyperalgesia, and allodynia. Negative signs involve sensory deficits (hypoesthesia and hypoalgesia), weakness, and reflex changes. Clinically, patients may complain of spontaneous ongoing pain (stimulus-independent pain), which is burning with intermittent shooting or electric shock-like (lancinating) sensations and/or by pain hypersensitivity evoked in response to stimuli (stimulus evoked pain) such as hyperalgesia and allodynia [97, 98] (Table 14.4).
Table 14.4
Symptoms and signs of neuropathic pain
Allodynia | Pain reported to normally nonpainful stimuli (light touch) |
Hyperpathia | Summation of painful stimuli induces |
Hyperalgesia | Increased response to a painful stimulus |
Hypoalgesia | Reduced response to a painful stimulus |
Hyperesthesia | Increased sensitivity to a stimulus |
Hypoesthesia | Decreased sensitivity to a stimulus |
Dysesthesia | Abnormal unpleasant sensation |
Paresthesia | Abnormal sensation |
Mechanisms of Neuropathic Pain
Studies in animal models describe a number of peripheral and central pathophysiological processes after nerve injury that would be the basis of underlying neuropathic pain mechanisms [26, 99]. A change in function, chemistry, and structures of neurons (neural plasticity) underlie the production of the altered sensitivity characteristics of neuropathic pain. Peripheral sensitization acts on the nociceptors, and central sensitization takes place at various levels ranging from the dorsal horn to the brain. In addition, abnormal interactions between the sympathetic and sensory pathways contribute to mechanisms mediating neuropathic pain [100].
Peripheral Processes in Neuropathic Pain
In the periphery, after an event that causes direct nerve damage, a pronounced local inflammatory response ensues. Around the site of injury nocisponsive primary afferent neurons (PAF), damaged tissue, infiltration of inflammatory cells (mast cells, macrophages, and other immunocompetent cells), the vasculature, and sympathetic terminals result in the release of an inflammatory “soup.” Upon PAF injury, the density and function of ion channels alter, responsible for abnormal patterns of electric impulses and afferent input to the dorsal horn. Non-synaptic interactions between neurons (neurons modifying activity in adjacent neurons) occur in the dorsal root ganglia and increase the already existing neuronal hyperexcitability. Additionally, following nerve damage, a phenotypic switch of Aβ-fibers may contribute to abnormal, pronociceptive actions following innocuous stimulation [101, 102].
Central Processes in Neuropathic Pain
Under normal circumstances, a painful stimulus results in the release of excitatory amino acids (EAA) (glutamate, aspartate), neurotrophins, and peptides (such as substance P, neurokinin A, and calcitonin gene-related peptide, CGRP) from the central terminals of nociceptive Aδ- and C-fibers in the dorsal horn. The EAAs (especially glutamate) interact with receptor subtypes (presynaptically and postsynaptic second-order neurons) including ionotropic receptors such as AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), and NMDA (N-methyl-D-aspartate) [102, 105]. Intensive or persistent noxious stimulation (repeated stimulation) by glutamate augment activation of the NMDA receptor (key for longer-lasting increased excitability of dorsal horn neurons) and produces central sensitization. As a result, subthreshold noxious input can activate postsynaptic second-order neurons. Central sensitization manifests as an exaggerated or amplified response to noxious stimuli (hyperalgesia), a spread of pain sensitivity beyond the site of injury (secondary hyperalgesia), and as a reduced threshold for elicitating pain. Furthermore, C-fiber input initiates a progressive increase in excitability during the course of the stimulus (windup of action potential discharge) [106]. Once this windup phenomenon is initiated, blockade of peripheral nociceptive input may not completely stop dorsal horn neurons from firing [5]. In response to peripheral nerve injury, Aβ-fibers (normally mediating sensations of vibration and touch but not pain) sprout into superficial layers of the dorsal horn to make inappropriate contacts with nociponsive neurons together with an escape from inhibitory interneurons and descending pathways. This rewiring may lead to the perception of an innocuous stimulation as noxious. Hence, low-threshold mechanical stimuli (light brushing of the skin) activating Aβ-fibers may now cause neuronal hyperexcitability resulting in pain (mechanical allodynia) [107]. After peripheral nerve injury microglia, oligodendrocytes, and astrocytes (central nervous system glial cells) in the dorsal horn are activated and release proinflammatory mediators that modulate pain processing by affecting either presynaptic release of neurotransmitters and/or postsynaptic excitability. Activated glia increase the release of nociceptive neurotransmitters and increase the excitability of nociceptive second-order neurons creating widespread pain changes in the spinal cord. Emphasizing the possible role of these cells could lead to new therapeutic strategies in the management of intractable neuropathic pain [108].
If the train of noxious stimuli persists, changes occur in gene regulation (induction of new proteins and effects on the levels of expression of existing proteins including dynorphin and substance P) in central neurons providing larger and longer-lasting modifications in dorsal horn and primary afferent neurons. These, possible irreversible, processes of transcription-dependent central sensitization may induce permanent phenotypic/morphological changes responsible for the persistent (and partially independent of peripheral noxious input) pain in patients [109, 110].
The NMDA receptor is responsible for both the induction, the initiation of hyperalgesia, and the subsequent maintenance of neuropathic pain. Although excitatory events have been long considered as the key event in neuropathic pain, loss of spinal inhibitory control (diminished release of inhibitory gamma-aminobutyric-acid: GABA) upon PAF input into the dorsal horn amplifies processes elicitating neuronal hyperexcitability. Another major inhibitory system, next to the GABAergic system, related to pain is opioid-receptor-mediated analgesia. In neuropathic pain, however, NMDA receptor activation increases excitation in the pain-transmitting systems. Thus, more opioids will be required for analgesia. Reducing excitations (NMDA antagonism) while increasing inhibition (opioids) may control neuropathic pain [106].
Descending Modulatory Pathways
Anatomic structures, including the periaqueductal gray area (PAG), the locus coeruleus, the nucleus raphe magnus, and several nuclei of the bulbar reticular formation give rise to descending modulatory pathways. These pathways may dampen or enhance the pain signal. The noradrenergic pathways, arising from the locus coeruleus play an antinociceptive role through activation of inhibitory dorsal horn localized α2-adrenorecepotors in inflammatory pain. The projections from the nucleus raphe magnus to the spinal cord are the major source of serotonin in the spinal cord. Although stimulation of the nucleus raphe magnus was shown to be antinociceptive in behavioral experiments, there is growing evidence that descending serotonergic pathways mediate both inhibition and enhancement of nociceptive processing in the dorsal horn [111]. The transmission of a pain signal from the periphery to the dorsal horn and supraspinal centers is a complex cascade of events. Although the transition from acute to chronic pain likely involves around activation of the NMDA receptor complex, phenotypic switches, structural reorganization in the dorsal horn, and loss of inhibitory circuits seem to underlie the most severe tractable form of neuropathic pain. Identification of molecular mechanisms of nociceptive signaling in the primary afferent neuron, the second-order neuron (dorsal horn), or beyond will provide a rational approach to neuropathic pain treatment and the selection of new targets for novel analgesic drug design [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







