Pain Assessment
Observer Pain Scores
In infants and preverbal children, assessment is dependent on observer evaluation of physiological and/or behavioural responses (Figure 12.1). As physiological measures are influenced by intercurrent illness and drug treatments, they are more often used in combination with behavioural responses in composite measurement tools (e.g. COMFORT is recommended for infants and children in intensive care; Table 12.1). The context of testing is important, as infant responses may overlap with other states of distress (e.g. hunger and fatigue). The FLACC scale scores five behaviours and has been validated for both acute procedural and postoperative pain (Table 12.2). Specific tools are also available for children with cognitive impairment (e.g. NCCPC, Non-Communicating Children’s Pain Checklist).
Figure 12.1 Assessment of pain in children requires assessment of many similar domains to those in adults, but may pose particular challenges, especially in non-specialist settings. A large number of measurement tools have been developed for children of different ages and for different clinical settings. Data from The Royal College of Nursing.
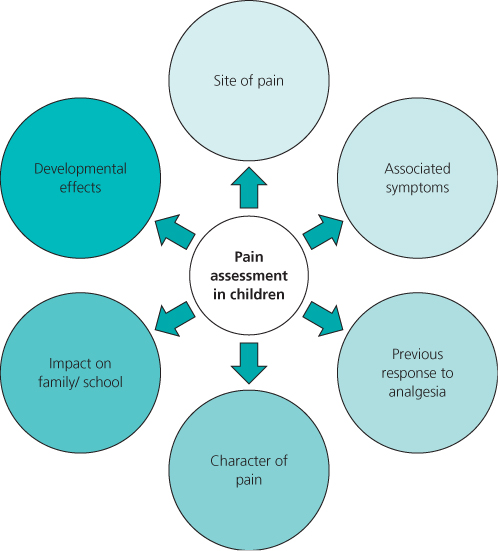
Table 12.1 COMFORT pain scale*
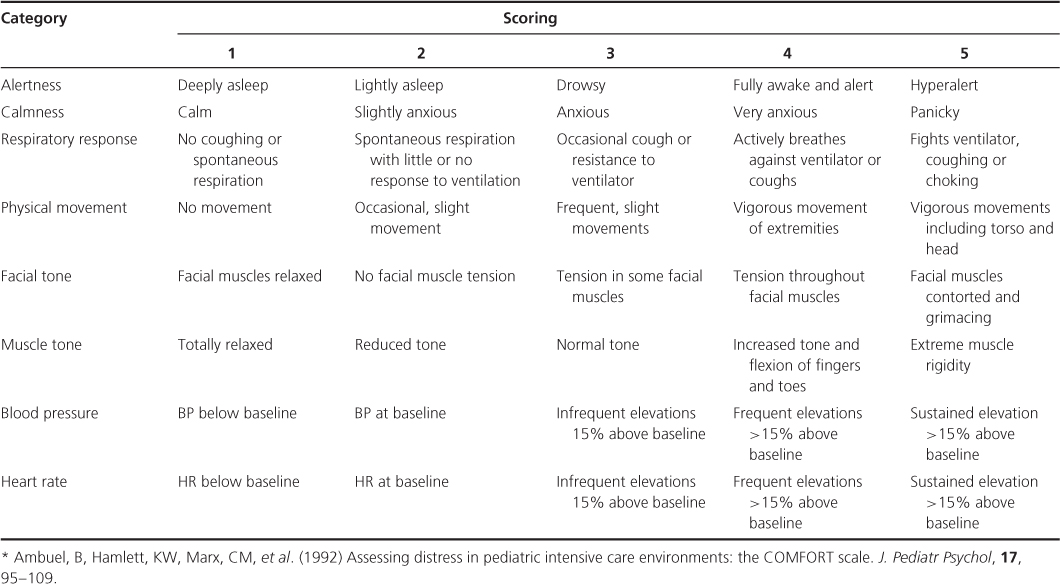
Table 12.2 FLACC pain scale*
Self-report
Children aged 4–5 years can use a graded pictorial scale (e.g. faces scales) but they are more likely to choose the extremes of the scale. In scales anchored with smiling or tearful faces, pain may be confused with other emotional states such as happiness/sadness or anxiety. In research trials, the Faces Pain Scale-Revised has been recommended for acute procedure-related, post-operative and disease-related pain for children aged 4–12 years. In clinical practice, standardised use of the same tool throughout an institution may be more important than which tool is chosen. Between seven and ten years children develop skills with measurement and can differentiate between the intervals on a scale and score their pain (e.g. 0–10 on a visual analogue scale). It is often not until 10–12 years of age that children can discriminate clearly between the sensory intensity and the affective emotional components of pain and report them independently.
Analgesia
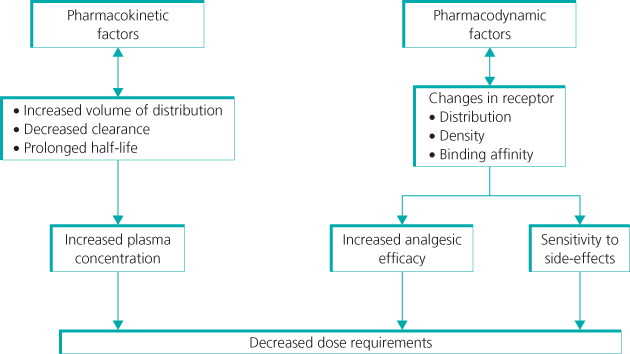
The efficacy and dose requirement for analgesics can vary significantly with age and depend on multiple factors (Figure 12.2). Clinical pharmacokinetic studies have established dose protocols that minimise side effects or toxicity. Pharmacodynamic effects also need to be considered, as age-related changes in receptor distribution and function may influence analgesic efficacy. For further details of analgesic use and dose recommendations see Further Reading and the British National Formulary for Children (http://bnfc.org/bnfc/).
Figure 12.2 Morphine dose requirements in early life. Multiple factors influence dose requirements for analgesics in early life. Changes in the pharmacokinetic handling of morphine result in higher plasma concentrations and a prolonged duration of action following single doses; to compensate, dose intervals need to be increased and infusion rates decreased. In addition, changes in the body’s response to morphine (i.e. pharmacodynamic effects) are altered by changes in receptor distribution and function, and as a result analgesic efficacy is achieved by lower plasma concentrations.
Opioids
Morphine can be given safely at all ages with appropriate dosing and monitoring. As individual requirements vary and there is no clear correlation between plasma morphine concentration and analgesia, doses need to be titrated against effect. In neonates and infants this is often achieved by intravenous infusion with nurse-administered additional boluses as required. With adequate instruction, children aged over 5–6 years can self-administer doses via a patient controlled (PCA) device.
The analgesic effect of codeine is wholly or mainly reliant on metabolism to morphine by the cytochrome P450 enzyme CYP2D6. In children with a genetically determined decrease in enzyme activity (up to 40% of a UK population), the efficacy of codeine is reduced. In addition, there is reduced CYP2D6 activity in neonates and infants.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








