The Edmonton classification system for cancer pain (ECS-CP)
The cancer pain prognostic scale
Classification of chronic pain of the international association for the study of pain (IASP)
Pain mechanism
Mixed pain
Regions involved (axis I)
Incident pain
Worst pain severity
Systems involved (axis II)
Psychological distress
Daily opioid dose
Temporal characteristics (axis III)
Addictive behavior
Emotional well-being
Pain intensity/time since onset of pain (axis IV)
Cognitive function
Aetiology (axis V)
Agreed upon definitions are of the utmost importance for description of patient cohorts in clinical studies, in clinical practice, and in order to understand for whom specific guidelines are developed. This is also necessary for making comparisons across studies and for drawing conclusions about medication or other treatment options. Stringent definitions of patient characteristics and observations are required to identify to which class or subclass the patient belongs (Cherny and Portenoy 2004; Hempel 1961).
The need for an internationally accepted classification system for cancer pain with a common language for use both in research and in clinical practice has been recognized in reviews, studies, and editorials (Caraceni and Portenoy 1999; Fainsinger and Nekolaichuk 2008; Kaasa et al. 2008; Mercadante et al. 2000b; Kaasa 2010). In order to succeed in being a frequently used tool in clinical practice, the system must be regarded as relevant, short, and applicable according to the given situation, i.e., prediction of pain relief. A major challenge, however, particularly so in palliative care, is that the system needs to be brief and sufficiently comprehensive. Items to consider include the clinical characteristics of the pain, the relationship to underlying pathological processes and pain mechanisms (i.e., visceral, neuropathic, idiopathic), other domains such as localization, as well as patient-related factors (i.e., socio-demographic factors, cognitive function, history of addiction, etc.).
Classification Systems for Cancer Pain
A review on cancer pain classification systems (Knudsen et al. 2009) identified three tools judged to be relevant for pain classification, all in the form of treatment evaluation tools. None of these were systematically developed or validated: The Opioid Escalation Index (OEI) is a classification system of opioid responsiveness used in the original (Mercadante et al. 1994), as well as in subsequent studies by the developers (Mercadante 1998; Mercadante et al. 2000a, b). The OEI is a measure of the patient’s opioid requirement combined with the level of pain intensity. Another system by the same author presented six prognostic groups for the likelihood of pain relief after pain treatment with NSAIDs and opioids (Mercadante et al. 1992). The factors included were number of days until achieving pain relief, the presence of incident pain, and the required dose of opioids. However, these scales were based on a retrospective grouping of patients after specific treatment regimens, thereby not providing a universal framework for cancer pain classification. The third of the nonvalidated systems, developed in 1994 (Cleeland et al. 1994), could be viewed as a treatment appropriateness evaluation tool, rather than a classification system. It compared the patient’s self-reported peak pain intensity with the most potent analgesic drug that was prescribed to the patient.
The review identified three classification systems that were labeled as formal systems. These tools encompass a set of domains and items which constitute a defined or standardized classification system intended for use across studies (Knudsen et al. 2009) and the tools were systematically developed and partially validated: the IASP Classification of Chronic Pain (International Association for the Study of Pain 1994; Bonica 1979), the Cancer Pain Prognostic Scale (CPPS) (Hwang et al. 2002), and the ECS-CP (Fainsinger and Nekolaichuk 2008; Fainsinger et al. 2005; Bruera et al. 1989, 1991; Nekolaichuk et al. 2005).
The IASP list of pain terms was first published in 1979 (Bonica 1979), later revised and extended twice (International Association for the Study of Pain 1994) as a result of expert opinions and clinical experience. In the IASP Classification of Chronic Pain for malignant and nonmalignant chronic pain syndromes, each clinical pain syndrome is assigned a code number based on five areas: (1) anatomical site, (2) organ systems of which abnormal functioning produces pain, (3) temporal characteristics, (4) pain intensity and time since debut, and (5) pain etiology. This provides important information about difficult malignant and chronic pain syndromes primarily based on physician’s examinations with pain intensity and duration being the only factors based on patients’ own report. The systematic literature review (Knudsen et al. 2009) identified only one clinical study in which the IASP system was used (Grond et al. 1996).
The same review identified only one study employing the CPPS: the original development study (Hwang et al. 2002). The CPPS is basically an index score, primarily based on self-report, that is used to dichotomize patients into good or poor prognosis for pain relief. The score is based on four domains: worst pain severity on an 11-point NRS scale (0–10), emotional well-being from the FACT-G (Functional Assessment of Cancer Therapy Scale) (Cella et al. 1993), daily oral opioid dose >60 mg, and the presence of mixed pain.
The ECS-CP, however, has gone through several, stepwise and systematic validation studies, and has recently been subject to a large, international validation study suggesting that the ECS-CP can predict pain complexity in a variety of countries and palliative care settings (Fainsinger et al. 2010). In the first version of the ECS-CP, called the Edmonton Staging System (ESS), patients with advanced cancer were classified as having a good, intermediate, or poor prognosis for successful pain treatment, based on their scores on seven domains: mechanism of pain, incident pain, previous opioid exposure, cognitive function, psychological distress, opioid tolerance, and past history of drug or alcohol abuse (Bruera et al. 1989). A subsequent study led to a dichotomization of the groups, good or bad prognosis for pain control (Bruera et al. 1995), while two of the factors, cognitive function and previous opioid consumption, were removed as they were not found to be independent predictors for achieving pain control.
In a later, regional multicentre study, aiming to test inter-rater reliability and predictive validity evidence, cognitive function was reintroduced based on expert opinions and literature reviews while tolerance was excluded due to interpretational difficulties (Fainsinger et al. 2005). The next validation study (Nekolaichuk et al. 2005) was performed to gather construct validity evidence to develop consensus definitions and to develop and evaluate the administration manual (Fainsinger et al. 2008). Input from national and international expert reviews by means of the Delphi techniques (Hasson et al. 2000) led to some revisions, with five domains (mechanism of pain, incident pain, addictive behavior, psychological distress, cognitive status) being included in the renamed ECS-CP.
The Present Recommendations for Cancer Pain Classification
In September 2009, an international expert meeting was arranged in Milan, Italy aiming to reach a consensus on how to assess and classify cancer pain (Kaasa et al. 2011). Meeting participants represented a wide range of disciplines (e.g., oncology, neurology, epidemiology, psychology, biostatistics, palliative care, public health, anesthesiology, and nursing) and were selected on the basis of their research and clinical expertise in cancer pain assessment and classification.
The panel was also representative of international research groups addressing cancer pain assessment and classification like the EPCRC (European Palliative Care Research Collaborative), a pan-European, EU funded translational research program (European Palliative Care Research Collaborative (EPCRC). http://www.epcrc.org/), PROMIS (Patient-Reported Outcomes Measurement Information System. http://www.nihpromis.org/default.aspx), IMMPACT (Initiative on Methods, Measurement and Pain Assessment in Clinical Trials. http://www.immpact.org/), CPOR-SG (Cancer Pain Outcome Research Study Group) (Apolone et al. 2006), and several associations involved in oncology and pain like the IASP (International Association for the Study of Pain. http://www.iasp-pain.org), ASCO (American Society of Clinical Oncology. http://www.asco.org/), ESMO (European Society for Medical Oncology) (European Association for Palliative Care (EAPC). http://www.eapcnet.org/), AIOM (Associazione Italiana di Oncologia Medica. http://www.aiom.it/), EORTC (European Organisation for the Research and Treatment of Cancer. http://www.eortc.be/), EAPC (European Association for Palliative Care. http://www.eapcnet.org/), SICP (Società Italiana Cura Palliativ. http://www.sicp.it/) and of international regulatory and health authorities, EMA (European Medicines Agency. http://www.ema.europa.eu/) and the WHO (World Health Organization. http://www.who.int/).
The expert panel suggested using the ECS-CP classification system as the template and international standard for assessment and classification of cancer pain (Kaasa et al. 2011). This is due to the stepwise and iterative process of development that has been followed in the development of the ECS-CP (Fainsinger and Nekolaichuk 2008; Fainsinger et al. 2005; Bruera et al. 1989, 1991; Nekolaichuk et al. 2005), which has also been the framework for instrument development within the EPCRC (Kaasa et al. 2008; European Palliative Care Research Collaborative (EPCRC). http://www.epcrc.org/) and other organizations (Patient-Reported Outcomes Measurement Information System (PROMIS). http://www.nihpromis.org/default.aspx; European Organisation for Research and Treatment of Cancer (EORTC). http://www.eortc.be/) with standardized procedures consisting of empirical data collection, literature reviews, expert consensus surveys, and patient focus groups and surveys (Kaasa et al. 2008; Hjermstad et al. 2009).
Furthermore, the ECS-CP is the only classification system that has been used in clinical work, albeit somewhat limited, because of the ongoing work both in Canada and internationally, with a large ECS-CP validation study led by the Canadian group (Fainsinger et al. 2010) and the multicenter study conducted by the EPCRC, the EPCRC-CSA (European Palliative Care Research Collaborative (EPCRC). http://www.epcrc.org/). The present version of the ECS-CP that has been launched under the acronym CPACS (Cancer Pain Assessment and Classification System) (Kaasa et al. 2010) contains four of the domains that have been identified by experts as important for pain assessment in palliative care (Hjermstad et al. 2008; Holen et al. 2006), namely, pain intensity, pain mechanism (± neuropathic pain), BTP, and psychological distress. However, based on forthcoming international clinical and validation studies, the content may be revised and developed further, as part of any dynamic tool development process in the twenty-first century (Kaasa et al. 2011).
Assessment of Cancer Pain
While the previous section emphasized the need for systematic consensus-based classification systems to optimize treatment and focused on the recent, promising progress in this respect, this section is devoted to the assessment of cancer pain in palliative care. A thorough assessment is crucial for a valid classification system as well as for day-to-day clinical management of pain.
Definitions and Concepts
Per definition, assessment is a synonym to estimation: the determination of importance, size, or value in various areas (http://www.merriam-webster.com/dictionary/symptom). Thus, pain assessment may be based on patients’ self-report obtained by answering single or multiple items (questions) that cover one or more pain domains.
A domain or dimension is the distinguished part of an abstract or physical space where something exists, is performed, or is valid (http://www.merriam-webster.com/dictionary/domain). In relation to cancer pain, pain intensity and BTP are domains of the pain symptom. For example, pain experts have recommended that cancer pain assessment comprises at least five key domains: pain intensity, temporal pattern, exacerbating/relieving factors, localization, and interference (Hjermstad et al. 2008; Holen et al. 2006).
An item is an entry in a list or one object in a collection of objects (http://www.merriam-webster.com/dictionary/item). In relation to measurement theory, it is based on the idea that the probability of getting an item correct is a function of a latent trait or ability. Thus, items for assessment of the various pain domains must be selected on the basis of their ability to serve as indicators of the specific pain domain, i.e., intensity, pain quality, BTP, etc., particularly so in clinical work. To enhance the clinical utility, the answer categories, time windows, and number of response options should be easily comprehensible and perceived as clinically relevant, once again a prerequisite for a correct classification.
Methods for Pain Assessment
Pain assessment may be based on information registered in the patients’ charts, proxy ratings, or observer-based scores by nurses or physicians if the patient is unable to respond verbally or in writing, due to trauma or different impairments. However, studies have shown that the correlation between patient-rated and observer-rated pain intensity decreased with increasing pain levels on a numerical rating scale from 0 to 10 (Ahlers et al. 2008; Banos et al. 1989). Thus, due to the subjective nature of the pain experience that is strongly influenced by individual variables (psychological, emotional, cultural, etc.), information on pain needs to be elicited directly from the patient whenever possible (Kaasa et al. 2008; Hjermstad et al. 2008) and then be combined with clinical findings and supplementary examinations. The use of subjective assessments, however, accentuates the importance of using a standardized assessment system to enhance the possibility to validly describe, quantify, and monitor pain at a given point or over time (Kaasa et al. 2008; Hjermstad et al. 2009).
Pain assessment has traditionally been performed by paper- and pencil-based questionnaires of various lengths for self-report, checklists of variable length, or in the form of interviews using closed or open-ended questions. Various mechanical or plastic devices, graphical charts, drawings, etc., are also used, but less often (Jensen 2003). The rapid technological development, however, now opens for pain registration by different electronic devices (palms, laptops, cell phones, direct web entries, etc.) that may facilitate the transfer of information from the patients’ charts to the bedside, yield immediate summated or index scores, and be readily available for any clinical or research purposes. It should be remembered, however, that these methods do not enhance the validity and clinical utility of the assessments per se, which are dependent on the format and selection of the questions that are presented to the patients.
Psychometric Requirements and Tool Development
All pain measures, regardless of mode of administration, must possess the necessary psychometric properties to provide reliable results. The psychometric properties encompass reliability issues; how consistently and reproducibly the instrument measures a symptom, different aspects of validity; if the instrument really measures what it purports to measure (face, content, criterion, construct validity), as well as sufficient sensitivity and specificity (Table 6.2). Ideally, pain assessment should be brief, precise, multidimensional, and specifically targeted to the patient population. There should also be a balance between the aspects of validity and brevity, especially so in frail patients. Furthermore, it should be remembered that even if a tool fulfills the statistical tests with respect to psychometric properties, it does not necessarily mean that it is valid for all populations in all situations, from diagnosis to the last stage of the disease. A systematic review examining 21 tools concluded that there is no ideal tool for general symptom assessment in cancer, based on the evaluation of psychometric properties, content, intended population, and practicality (Kirkova et al. 2006), a conclusion that also pertains to pain assessment tools.
Table 6.2
Criteria for the ideal symptom assessment tool for self-report
Characteristics | Requirements |
|---|---|
Acceptable | Easy and quick to complete, for patients and clinicians |
Perceived as clinically useful, for patients and clinicians | |
Easy and quick to score | |
Comprehensive with relevant content | Comprehensive symptom assessment, prevalence, severity and distress |
Relevant and sufficient for decision making, targeted, relevant timeframe | |
Applicable for research | |
Flexible | Adaptive to changes and development |
Applicable in various formats (paper, electronic, plastic) | |
User-friendly | Brief, easy to understand, easy to score appealing lay-out |
Unequivocal questions and answers | |
Psychometrically sound | Reliability: precise, reproducible, stable, repeatable, good internal consistency |
Validity: measures what’s intended, appropriate, meaningful and useful for a specific purpose | |
•Sufficient face and content validity | |
•Sufficient criterion validity (concurrent and predictive) | |
•Sufficient construct validity (discriminative and convergent) | |
Accuracy: correlates well with similar tools | |
Sensitivity: identifying present symptoms | |
Specificity: excluding symptoms that are not present | |
Responsiveness: detects with-in patient changes over time |
A major problem in relation to cancer pain assessment, in general oncology as well as in palliative care, is the abundance of tools, poorly defined concepts, huge variations in the answer options, and ambiguity in the interpretation of scores, which in turn creates confusion and prohibits comparison across studies. A review showed that 24 different adjectives were used to anchor the extreme scale values in 54 studies aiming to compare unidimensional scales for assessment of pain intensity (Hjermstad et al. 2011). Whether the variability in anchors and response options directly influences the numerical scores is a question that needs to be empirically tested, but also something that in itself calls for a standardization of assessment methods. A review on the contents of pain assessment tools, covering the period from 1966 to 2003 (Holen et al. 2006), showed that 80 different tools assessing pain by one or more items were used for self-report of pain in palliative cancer care. A subsequent follow-up review identified 11 new pain assessment tools developed for use in palliative care in the next 4 years (Hjermstad et al. 2008). Of these 11, 9 were multidimensional, and 3 of the 5 highest ranked domains in Holen’s review (Holen et al. 2006): intensity (rank 1), treatment/relief/exacerbation (rank 3), and localization (rank 4) were included in seven, six, and five of the tools, respectively. Only one, an ad hoc inventory for clinical practice, included all five dimensions (Gutgsell et al. 2003).
Another problem that is related to the steady flow of new tools is the fact that many tools are developed to examine a small and specific area of interest, i.e., pain beliefs, information, prescription routines, etc. (Hjermstad et al. 2008). This limits the usefulness of the tools that are used only once or maybe in a very limited number of studies (Hjermstad et al. 2008). Consequently, there is often a need for additional instruments in clinical studies to obtain a detailed pain assessment, which in turn increases the burden on patient and staff. The instrument package may be perceived as cumbersome by both parties, which may reduce the use, compliance, and validity, and the “vicious circle” of unsystematic symptom assessment is complete. Furthermore, many new tools have been developed without adhering to the recommended guidelines for tool development to ensure adequate psychometric properties, such as the internationally accepted EORTC methodology (Sprangers et al. 1998). Only 2 of 11 instruments identified in a review were extensively validated or cross-culturally tested (Hjermstad et al. 2008). To meet the methodological requirements for development of symptom tools, the work of the EPCRC has been conducted in a systematic, stepwise manner with systematic literature reviews, expert opinions, patient input, empirical testing and validation, and international consensus processes, as previously described in detail (Kaasa et al. 2008; Hjermstad et al. 2009; Haugen and Kaasa 2010; European Palliative Care Research Collaborative (EPCRC). http://www.epcrc.org/). This process has been followed in the development of other assessment tools as well, e.g., within the PROMIS (Patient-Reported Outcomes Measurement Information System (PROMIS). http://www.nihpromis.org/default.aspx) and the EORTC (European Organisation for Research and Treatment of Cancer (EORTC). http://www.eortc.be/).
In order to optimize pain assessment, clear definitions and conceptualizations of the relevant domains to be included are necessary, as strongly emphasized in the NIH consensus statement on pain, depression, and fatigue some years ago (Consensus Development Program and National Institutes of Health 2002). The clinical relevance and face validity are key aspects for clinical use and standardization, and despite patients’ involvement in tool development being emphasized (Food and Drug Administration 2006; Burgers et al. 2004; DeWalt et al. 2007), this step is often bypassed in the development of new tools. An example of the conceptual framework used to ensure a uniform use of concepts in the development process within the EPCRC is displayed in Fig. 6.1.
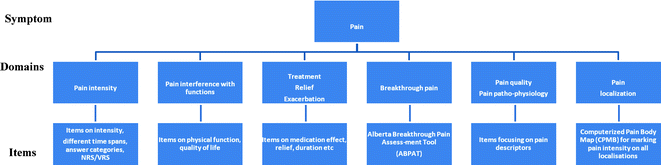

Fig. 6.1
The conceptual framework used for assessment of pain in the EPCRC data collection study The figure displays the structural framework used by the EPCRC (European Palliative Care Research Collaborative (EPCRC). http://www.epcrc.org/) with the overall symptom pain, being divided into different domains: i.e., intensity, breakthrough pain which are assessed by several items
Pain Assessment Tools in Palliative Cancer Care
Many comprehensive symptom tools and symptom distress tools like the 31-item Rotterdam Symptom Checklist (RSCL) (de Haes et al. 1990), the 13-item core version of the MDASI (M. D. Anderson Symptom Inventory) (Cleeland et al. 2000), and the 32-item Memorial Symptom Assessment Scale Short Form (MSAS-SF) (Chang et al. 2000) contain one or more items on pain (Table 6.3). Together with self-reporting tools on general QoL or health-related QoL such as the SF-36 (Ware 1993), the EORTC QLQ-C30 (Aaronson et al. 1993), the shorter version EORTC QLQ PAL-15 for palliative care patients (Groenvold et al. 2006), the FLIC (Schipper et al. 1984) and the FACT-G (Cella et al. 1993), these are in frequent use in cancer (Victorson et al. 2008). All these inventories contain highly prevalent symptoms like pain, depression, nausea, and fatigue, and the items are most often scored on verbal rating scales (VRS) with 4–6 answer categories with different time frames (Table 6.3). The pain items may be in the form of single item scores, form multi-item pain scales, or be part of summated symptom index scores. Depending on the actual tool, the intensity, frequency, and impact of symptoms on various functions may be assessed.
Table 6.3
Description and content of frequently used multidimensional tools used for assessment of pain in palliative cancer care
Name of toola | Author, pub yr | Pain dimensions in toolb | No of pain items | Uni-dimensional pain or pain scale | Scale, answer categories for pain items in tool | Time line for pain items |
|---|---|---|---|---|---|---|
General symptom tools | ||||||
ESAS | Bruera et al. (1989) | Int | NRS-11, i.e., 0 no pain – 10 worst possible pain | Right now | ||
MDASI | Cleeland et al. (2000) | Int | 1 | Uni | NRS-11: 0 not present – 10 as bad as you can imagine according to question | During the past week |
MSAS-SF | Chang et al. (2000) | Int, Treat, + pain syndromes | 1 | Uni | Presence of symptom:, dichotomous Y/N Frequency: VRS-4: 1 rarely– 4 almost constantly Severity: VRS-4: 1 slight – 4 very severe Distress: VRS-5: 0 not at all – 4 very much | During the past week |
Quality of life tools | ||||||
EORTC QLQ-C30 | Aaronson et al. (1993) | Int, inf | 2 | Pain scale | VRS-4, 1: “not at all” – 4: very much | During the past week |
EORTC QLQ-PAL 15 | Groenvold et al. (2006) | Int, inf | 2 | Pain scale | VRS-4, 1: “not at all” – 4: very much | During the past week |
FACT-G | Cella et al. (1993) | Int | 1 | Single item | VRS-5, 0: “not at all” – 4: very much | Past 7 days |
FLIC | Schipper et al. (1984) | Inf, Bel | 2 | |||
SF-36 | Ware (1993) | Int, inf | 2 | Bodily pain scales | Last week | |
Pain tools | ||||||
BPI-SF | Daut et al. (1983) | Int, Loc, Inf, Treat, Qual | 15 | Intensity scales, 4 items | Int: NRS-11: 0 no pain – 10 pain as bad as you can imagine Loc: body map Inf: related to seven areas: general activity, mood, walking ability, normal work, relations with other persons, sleep, enjoyment of life, NRS-11, anchors depending on area | Int: worst and least pain past 24 h + average and present |
SF-MPQ | Melzack (1987) | Int, Loc, Qual, Temp | 23 | 3 scales: affective, sensory, total | Scales: VRS-4: 0 none – 3 severe Int: VAS 100 mm: no pain – worst possible pain, PPI: VRS-5: 0 no pain – 5 excruciating | Int: past week, present |
Generally speaking, however, QoL tools, general symptom checklists, or distress inventories are less well suited for close clinical follow-up, assessment of fluctuating symptoms such as pain, and monitoring of treatment effect. This is first and foremost related to the time frame, which most often refers to the past week, up to the past 4 weeks (Table 6.3), but also to the fact that some arithmetic may be necessary to calculate clinically meaningful scores for items and scales, limiting the usefulness of the tools in daily clinical practice. Because pain intensity probably is the most clinically relevant dimension of the pain experience, one should be cautious to use combination scores to guide treatment, without specifically monitoring pain intensity per se. Combining pain intensity scores with, e.g., pain interference may be less relevant in clinical settings as it may obscure the actual scores of each domain (Jensen 2003; Fayers et al. 2011). Research has shown that patients may not be able to distinguish between functional impairment due to pain and impairment that is due to other causes (Stenseth et al. 2007).
Nevertheless, it is important to remember that a complex pain experience requires a multidimensional assessment. Well-validated instruments like the Brief Pain Inventory (BPI) (Daut et al. 1983) or the McGill Short Form questionnaire (Melzack 1987) are recommended for a more comprehensive, multidimensional pain assessment in cancer (Kaasa et al. 2008; Jensen 2003; Caraceni et al. 2002). Both tools are specific pain tools (Table 6.3), are frequently used in cancer clinical trials and follow-up studies, and have been translated into and validated in several languages.
The BPI (Daut et al. 1983) was designed to assess pain intensity and to what extent pain interferes with normal activity. The intensity scale contains four items measuring worst and least pain during the past 24 h, and average and present pain intensity, all scored on NRS-11 (Table 6.3). Pain interference is assessed in relation to seven areas of daily life (general activity, mood, walking ability, normal work, relations with other persons, sleep, enjoyment of life). However, studies using the BPI have observed that the BPI interference scores are higher in patients with deteriorated functional performance compared to patients with close to normal functional performance, regardless of the pain intensity scores, and that the interference items do not function optimally from a psychometric point of view (Holen et al. 2008; Radbruch et al. 1999).
The McGill Pain Questionnaire (MPQ) (Melzack 1975) is a measure of the subjective pain experience, across sensory, affective, and evaluative dimensions of pain. It has been found to discriminate well between acute and chronic pain and to be sensitive enough to detect differences in pain relief. However, it uses 78 words to describe pain quality and requires at least 5–10 min to complete. Thus, the shorter version (SF-MPQ) (Melzack 1987) is a more efficient measure of pain for clinical assessment and research purposes, well validated in various patient populations (Grafton et al. 2005; Melzack and Katz 2001) and is widely used in cancer clinical settings. The SF-MPQ consists of 15 descriptors (11 sensory; 4 affective) which are rated on an intensity scale as 0 = none, 1 = mild, 2 = moderate, or 3 = severe. Three pain scores are derived from the sum of the intensity rank values of the words chosen for sensory, affective, and total descriptors, respectively. The SF-MPQ also includes the Present Pain Intensity (PPI) index of the standard MPQ and a visual analogue scale (VAS) for assessment of pain intensity (Table 6.3).
The Edmonton Symptom Assessment Scale (ESAS) (Bruera et al. 1991) is probably the most frequently used and well-known self-reporting tool for assessment of symptoms in palliative care (Nekolaichuk et al. 2008). It has been validated in different samples and nationalities and against other instruments like the FACT-G (Cella et al. 1993) and the MSAS (Chang et al. 2000). The ESAS consists of the nine frequent cancer symptoms (pain, nausea, tiredness, etc.) (plus an optional tenth) that are scored on a Numerical Rating Scale (NRS-11) (0 = not at all, no symptom, best, 10 = worst possible) with a time frame referring to the present situation (now) (Table 6.3). Most studies demonstrate high compliance (Nekolaichuk et al. 2008), although qualitative research has shown that patients report difficulties in relation to terminology and numerical rating assignments, implying that more extensive validation and modifications of the tool may be necessary (Nekolaichuk et al. 2008; Watanabe et al. 2009). However, the tool is easy to score, yields immediate results, and is probably most effective for immediate screening of clinical symptoms.
Reviews and recommendations agree on the use of single-item unidimensional tools, such as Numerical Rating Scales (NRS), VRS, and VAS, for assessment of pain intensity in clinical settings (Jensen 2003; Breivik et al. 2008; Caraceni et al. 2002; Dworkin et al. 2005). Among the unidimensional tools, the VAS is by far the most frequently used scale (Caraceni et al. 2005; Jensen 2003). The NRS and the VAS are shown to be more powerful in detecting changes in pain intensity than a VRS (Kaasa et al. 2010; Jensen 2003; Breivik et al. 2008; Caraceni et al. 2002), but frail, elderly patients and the cognitively impaired may have difficulties interpreting the VAS (Jensen 2003; Caraceni et al. 2002; Chibnall and Tait 2001). A short description of unidimensional scales is presented in Table 6.4.
Table 6.4
Properties of numerical rating scale, verbal rating scale and visual rating scale
Numerical rating scale – NRS | Commonly from 0 to 10 (NRS-11) or 1–10 (NRS-10) Usually, only the two extreme categories are labeled, e.g., “no pain at all” and “worst imaginable pain” NRS may be called a VNRS/VNS when the scale is explained or shown on paper to the patient who responds by indicating a number |
Verbal rating scale – VRS | Ordered categorical scale with each response option consisting of adjectives. For different levels of pain intensity, “no pain,” “mild pain,” “moderate pain,” “severe pain,” “extreme pain,” and the “most intense pain imaginable” form a 6-category VRS scale (VRS-6) VRS scales are commonly of lengths 4–7. The adjectives are scored by assigning numbers (0–6) to each response option The scale may also be called VPS (verbal pain scale), VDS (verbal descriptor scale) or SDS (simple descriptor scale) |
Bipolar – VRS | A VRS that goes from, e.g., “very much worse” to “very much better” Usually with a central neutral option (e.g., “no change”), but may also have no neutral option thereby forcing decisions by using an even number of response options. Bipolar scales, therefore, usually have 3, 5, or 7 options |
Visual analogue scale – VAS | Consists of a straight line, usually 0–100 mm, with the extreme categories labeled as for NRS. The distance measured from the “no pain” end to the patient’s mark is the VAS score Often graduated with labeled marks indicating tens (10, 20, 30, etc) or with unlabelled marks for the units |
To ensure a uniform way of assessing pain, the expert group from the Milan meeting proposed that similar methods for cancer pain assessment should be used whenever possible, both in clinical practice and in clinical research (Kaasa et al. 2010). However, this does not preclude specific considerations with respect to the actual patient population. On the one hand, it is obvious that certain population characteristics have to be considered, such as age, frailty, literacy level, cognitive impairment, etc. For example, the higher number of errors on the VASs with increasing age and other impairments makes this scale less applicable in the cognitively impaired, as documented in the literature (Jensen 2003; Caraceni et al. 2002; Chibnall and Tait 2001). This is also in line with a recent letter based on a study comparing NRS and VRS, emphasizing the need to be selective in the use of scales for clinical use (Ripamonti and Brunelli 2009). However, because the psychometric properties largely depend on certain basic characteristics, the selection of scales is better guided by specific consensus-based recommendations rather than left to the judgment of the individual clinicians. This also means that the same methodology (scale, wording, time frame, and format) should be applied when assessing pain over time in the same patient population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




