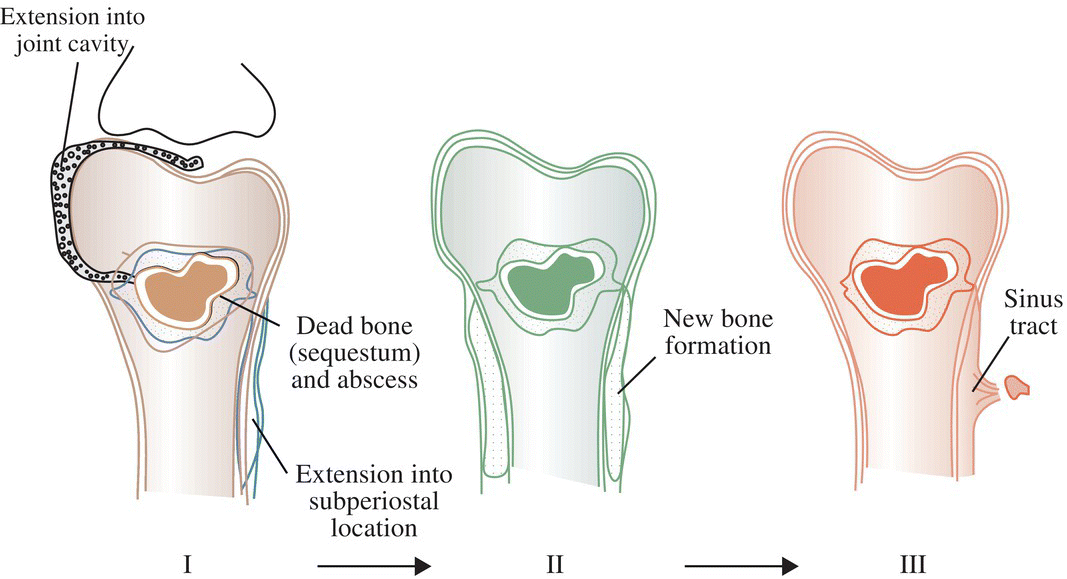
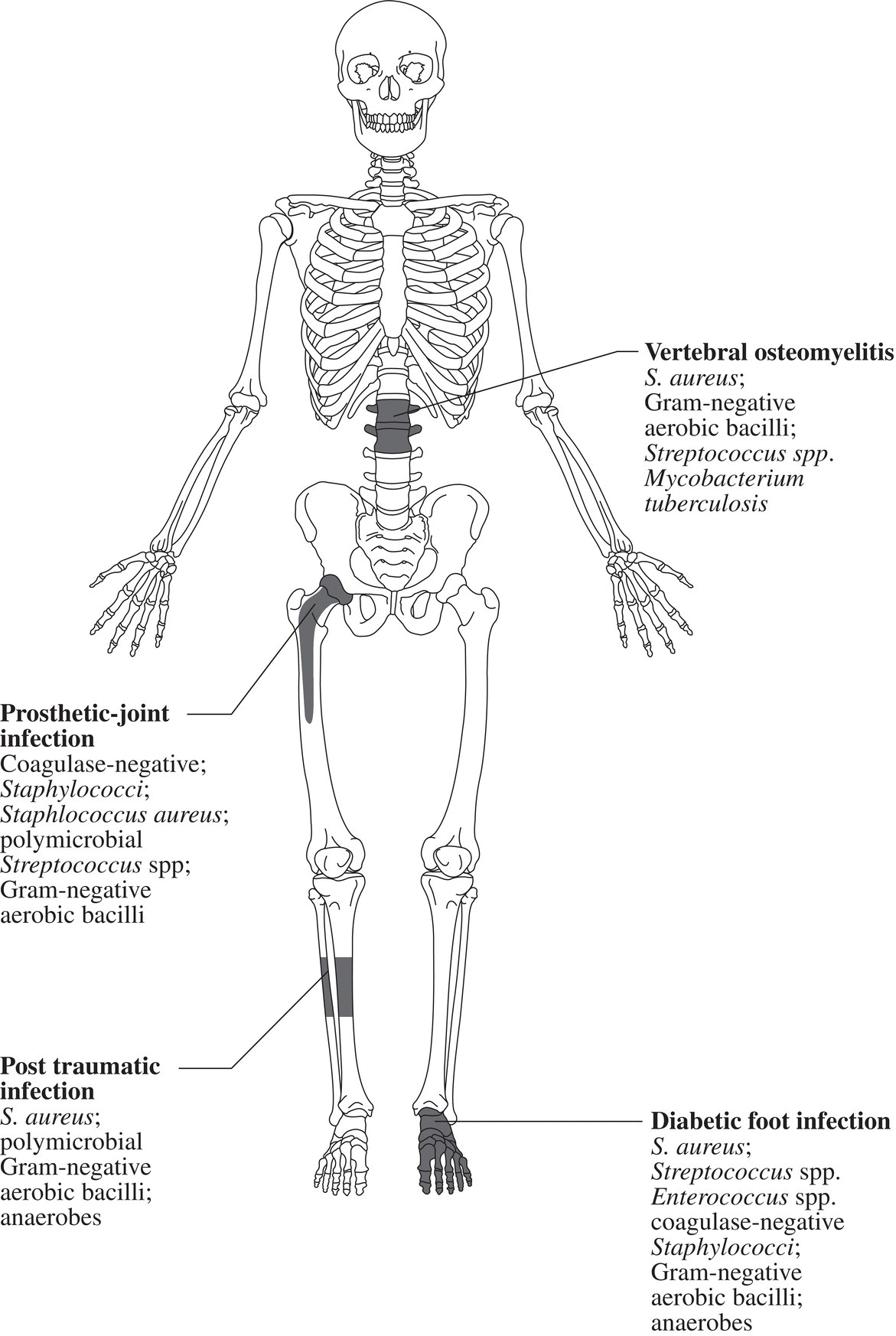
Ian T. Ferguson and Christopher R. Carpenter Department of Emergency Medicine, Washington University School of Medicine, St. Louis, MO, USA Osteomyelitis is an infectious, inflammatory process that results in bony destruction. The infection can be isolated to the cortex or involve the periosteum and surrounding soft tissues.1 The symptoms of acute osteomyelitis are typically noted over weeks, while chronic osteomyelitis (Figure 33.1) can evolve over months or years. Lew and Waldvogel divide osteomyelitis etiologies into three categories: (i) hematogenous osteomyelitis; (ii) direct inoculation from trauma, surgery, prostheses, or soft tissue spread; or (iii) vascular insufficiency seen most often in diabetes.2 Risk factors for osteomyelitis include intravascular catheters, intravenous drug abuse, and nonbone foci of infections such as cellulitis, cutaneous abscesses, and urinary tract infections.3 Hematogenous spread to the long bones is the most common etiology in children with more than half of cases occurring in children less than 5 years old. Risk factors include indwelling catheters, skin or soft tissue infection, or abnormal urinary anatomy.4 Sickle cell disease is another risk factor for osteomyelitis in children with an estimated incidence of 8 cases per 100,000 children per year. Direct inoculation is more common in adults, often as a result of trauma which can complicate 25% of open fractures.4 Figure 33.1 The progression of chronic osteomyelitis. Phase I: An area of devascularized dead bone is sequestered into an abscess cavity. Note that extension of intramedullary infection into an intracapsular location can result in septic arthritis, while progression into a periosteal location may produce periosteal elevation. Phase II: New bone formation results from periosteal elevation. Phase III: Extension of the abscess and necrotic material through cortical bone creates a fistula. Reproduced from Jauregui LE et al. Diagnosis and management of bone infections (1995)2 Staphylococcal species are the most commonly isolated organisms, but the specific infective agent depends upon the site of infection, whether the spread is hematogenous or contiguous, and whether the joint is prosthetic or native (Figure 33.2). Osteomyelitis is a heterogeneous disease and no uniform treatment algorithm exists although antibiotic therapy is the mainstay of treatment in all cases.5 The general approach to management involves a combination of intravenous antibiotics often with surgical debridement, followed by an additional extended course of oral antibiotics.6 Local antibiotic delivery using antibiotic‐containing beads or cement spacers may be used in some cases.7 Figure 33.2 The microbiology of osteomyelitis. The microorganisms of osteomyelitis in order of prevalence from high to low by site of infection. (Reproduced from [1]/With permission of Elsevier.) One frequently encountered emergency department scenario in which osteomyelitis is a concern is foot ulcers. Foot ulcers can be venous, arterial, or diabetic, and the distinction can be challenging.8 Generally, venous ulcers are proximal to the malleoli with irregular borders, whereas arterial ulcers involve the toes or shins with a pale and punched‐out appearance. By comparison, diabetic ulcers typically occur in areas of increased pressure, such as the soles. Charcot neuropathy is a severe complication of peripheral neuropathy and can mimic diabetic osteomyelitis with a very similar clinical presentation, but the management for the two conditions is vastly different. Charcot neuropathy is treated with a cast and non‐weight‐bearing status rather than antibiotics and debridement. The prevalence of Charcot neuropathy in diabetic patients ranges from 0.08% to 13% in high‐risk patients.9 Distinguishing Charcot neuropathy from diabetic osteomyelitis is important for emergency physicians since catastrophic bony collapse in the ankle joint can occur in a short timeframe in Charcot neuropathy.10 Despite the fact that osteomyelitis of the foot or ankle is the primary or secondary reason for 75,000 hospitalizations in the United States each year, clinicians often underestimate the likelihood of diabetic foot osteomyelitis.11 The pretest probability for osteomyelitis in a diabetic foot wound ranges from 15% to 20%.12 Foot‐related complications account for 20% of diabetes‐related admissions in North America8 and as many as 15% of patients with diabetic foot ulcers ultimately require amputations. One study found that, over a 3‐year period, 61% of diabetic foot ulcers are likely to recur, 10% of ulcers will lead to amputation, and the overall survival rate for diabetic patients with foot ulcers was only 72% (compared to 87% for age‐ and gender‐matched diabetic control patients).13 The presence of diabetic foot osteomyelitis increases the risk of amputation and the 30‐day postamputation perioperative mortality ranges from 7% to 15%.14–16 Can history, physical exam, or labs, accurately differentiate acute osteomyelitis from other causes of musculoskeletal or infectious symptoms? One 2008 diagnostic meta‐analysis concluded that no single laboratory or physical exam finding can significantly decrease the likelihood of diabetic osteomyelitis17 (Table 33.1), although using a combination of findings may significantly reduce the negative likelihood ratio (LR−). A case‐control study combining ulcer depth >3 mm with C‐reactive protein (CRP) > 3.2 mg/dL (or erythrocyte sedimentation rate [ESR] > 60 mm/hour) reported LR− 0.009 for osteomyelitis.18 Ulcer size >2 cm, bone exposure, a positive probe‐to‐bone test, a Wagner grade >2, or ESR >70 mm/hour each significantly increases the likelihood of diabetic osteomyelitis (Table 33.1).19–23 Table 33.1 Diagnostic test characteristics for diabetic osteomyelitis Source: Data from [19]. Note: Wagner grading scale: A systematic review on the utility of the probe‐to‐bone test in diagnosing diabetic osteomyelitis reported a pooled LR+ of 5.12 and a LR− 0.16.24 These systematic review authors concluded, in conjunction with the current international working group on the diabetic foot guidelines,25 that the probe‐to‐bone test should be used to rule in osteomyelitis in high‐risk patients (positive predictive value [PPV] >90% if pretest probability >60%), and used to rule out osteomyelitis in low risk, outpatient settings (negative predictive value [NPV] >95% at pretest probability below 20%). To conduct this test, the clinician should use either a sterile blunt end probe or mosquito forceps to explore the entire depth of the wound: a positive test is one in which a grinding sensation is appreciated as the object is moved across the bony surface (Figure 33.3).26 Laboratory values that have been investigated to aid the diagnosis of osteomyelitis include ESR, CRP, white blood cell
Chapter 33
Osteomyelitis
Background
Clinical question
Diagnostic test
LR+ (95% confidence interval)
LR− (95% confidence interval)
Bone exposure
9.2 (0.57–146)
0.70 (0.53–0.92)
Probe to bone test
6.4 (3.6–11)
0.39 (0.20–0.76)
Ulcer area >2 cm2
7.2 (1.1–49)
0.48 (0.31–0.76)
Ulcer inflammation
1.5 (0.51–4.7)
0.84 (0.56–1.3)
Clinical judgment
9.2 (0.57–147)
0.70 (0.53–0.92)
Wagner >2
5.5 (1.8–17)
0.54 (0.30–0.97)
ESR >70 mm/hour
11.0 (1.6–79)
0.34 (0.06–1.9)
Swab culture
1.0 (0.65–79)
1.0 (0.08–13)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






