Orthopedic
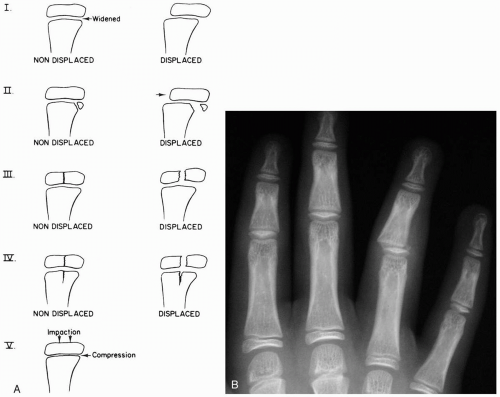
15-1 Salter-Harris Classification
Robert Hendrickson
Clinical Presentation
Patients with fractures described by the Salter-Harris (SH) classification system present with pain, tenderness, and possibly swelling. Injuries may occur after minimal trauma, because the growth plate is II to V times weaker than the surrounding bone.1 SH II-V fractures should be readily recognizable on radiographs. An SH 1 fracture may not be detectable on radiographs, and conservative therapy for a presumed fracture may be needed.1,2
Pathophysiology
Fractures may occur via direct trauma or rotational pressure. The SH classification of fractures is a system of grouping childhood fractures that involve the epiphyseal growth plate (physis). SH I is a fracture through the growth plate only. These fractures may or may not be recognizable on radiographs. In SH II, the fracture is through the metaphysis as well as the physis. SH III fractures are intraarticular fractures through the physis as well as the epiphysis. SH IV fractures traverse both the epiphysis and the metaphysis. SH V is a crush injury to the growth plate and is noted as a compression of the physis.1,2
Diagnosis
Diagnosis is suspected by recognition of clinical symptoms and confirmed by radiography. Comparison views of the contralateral extremity are sometimes helpful in detecting fractures.
Clinical Complications
Complications include premature growth plate closure, bony growth arrest, angular deformity, nonunion, and chronic pain.2 Prognosis is indirectly related to classification number. For example, SH V has a worse prognosis than SH IV, which has a worse prognosis than SH III, and so on.
Management
If a patient has pain and tenderness of a growth-plate area, the involved part should be immobilized with a splint, and follow-up with an orthopedist or primary doctor should be sought, because SH I fractures are extremely difficult to detect on radiography and usually are diagnosed clinically. Patients with SH II-V fractures also require splinting and an orthopedic consultation.
REFERENCES
1. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician 1992;46:1180-1184.
2. Rogers LF, Poznanski AK. Imaging of epiphyseal injuries. Radiology 1994;191:297-308.
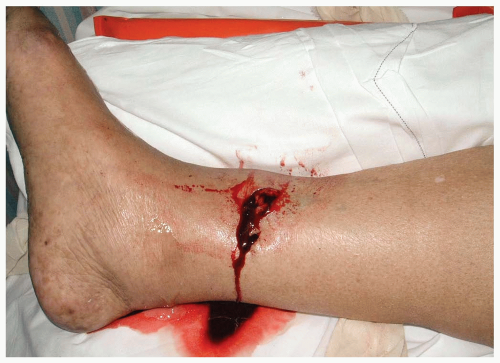
15-2 Open Fractures
Colleen Campbell
Clinical Presentation
Patients with open fractures (OFs) present with pain in the involved extremity, along with an overlying laceration or puncture wound.
Pathophysiology
An OF is a fracture in which there is an overlying disruption of the normal skin barrier, with a resultant exposure or tract from the skin to the fracture or its hematoma. One classification of open fractures is as follows. Grade I is a clean puncture wound of less than 1 centimeter. Grade II is a laceration of less than 5 cm that does not involve extensive soft tissue injury or loss. Grade III injuries have extensive soft tissue damage by crush injury or involve extensive loss of tissue. Grade IIIA injuries have adequate soft tissue coverage of bone; IIIB injuries have associated periosteal stripping and bone exposure; and Grade IIIC injuries involve extensive arterial injury.1
Diagnosis
The diagnosis of an OF is made on the basis of a careful history and physical examination, along with radiographic evaluation of the involved underlying bone. A careful examination and documentation of the neurovascular status of the affected limb is essential.1
Clinical Complications
OFs are complicated by infection, osteomyelitis, nonunion, and delayed union. The underlying bone and soft tissue may be devascularized to the extent that amputation is necessary. Because of the large forces that cause OFs, there is often associated myonecrosis and rhabdomyolysis.1
Management
Initial treatment of OFs involves immobilization and emergency orthopedic referral. Antibiotics effective against skin organisms should be given intravenously in the emergency department (ED). First-generation cephalosporins are the drugs of choice. Tetanus prophylaxis should be provided if indicated. Débridement of the wound, followed by copious high-pressure irrigation, should be done in a timely manner in the ED if surgery will be delayed for longer than 1 hour.1 Sterile dressings should then be applied. Treatment of OFs involves high-pressure irrigation and débridement in the operating room, as well as fracture fixation. Intramedullary nails are used with increasing frequency in the treatment of open tibia and femur fractures. External fracture fixation may be necessary if there is a large amount of soft tissue injury or if hemorrhage must be controlled quickly, as in the case of pelvic fractures.1
REFERENCES
1. Musgrave DS, Mendelson SA. Pediatric Orthopedic Trauma: Principles in management. Crit Care Med 2002;30[11 Suppl]:S431-S443.
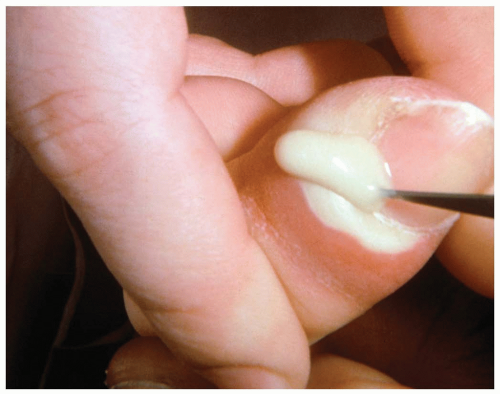
15-3 Paronychia
Jennifer Harris
Clinical Presentation
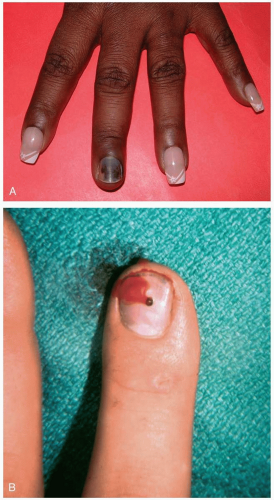
Patients with paronychia present with acute or chronic pain and tenderness of the perionychium, the fingers, or the toes. Acutely, patients report symptoms arising spontaneously or after trauma or manipulation of the nail bed. Chronic symptoms usually are present for longer than 6 weeks and may be episodic, typically occurring after exposure to water or a moist environment.1
Pathophysiology
Paronychia is a localized, superficial infection or abscess of the epidermis bordering the nails that develops when a disruption occurs between the seal of the proximal nail fold and the nail plate. Acute paronychia is caused by trauma, nail biting, finger sucking, aggressive manicuring, or a hangnail. The most common offending organism is Staphylococcus aureus, followed by Streptococcus and Pseudomonas species, but gram-negative organisms, herpes simplex virus, and yeast also have been reported.1 In chronic infections, separation of the cuticle from the nail plate occurs secondary to overexposure to water, solvents, or chemicals. The infecting organism is typically Candida albicans.2 In addition, metastatic cancer, subungual melanoma, and squamous cell carcinoma can manifest as chronic paronychia.1
Diagnosis
Diagnosis of acute or chronic paronychia is made by physical examination and history. Because chronic paronychia typically is caused by C. albicans (95% of cases), a wet mount with potassium hydroxide from a scraping or sample of the purulent drainage may reveal hyphae.1
Clinical Complications
Neglected paronychia, although painful, has few long-term complications. If chronic paronychia does not resolve with appropriate therapy, unusual and potentially serious causes such as malignancy must be explored.1
Management
Acute paronychia in which abscess formation has not occurred may resolve with conservative treatment (e.g., warm-water soaks). Antibiotics, if used, should cover S. aureus as well as anaerobes; amoxicillin-clavulanate potassium (Augmentin) or clindamycin (Cleocin) is the drug of choice. Once abscesses have formed, incision of the nail fold to promote drainage is required to clear the infection.1 The treatment of chronic paronychia should include avoidance of exposure to the offending agent (water, solvents) and application of topical antifungal drugs and steroids. If secondary bacterial infection is suspected, antibiotic ointment or oral agents should be added to the regimen.2 Long-standing paronychia may require more invasive treatment (e.g., removal of the entire nail for adequate drainage) but usually responds once addressed.
REFERENCES
1. Rockwell PG. Acute and chronic paronychia. Am Fam Physician 2001;63:1113-1116.
2. Rich P. Nail disorders: diagnosis and treatment of infectious, inflammatory, and neoplastic nail conditions. Med Clin North Am 1998;82:1171-1183.
15-4 Felon
Colleen Campbell
Clinical Presentation
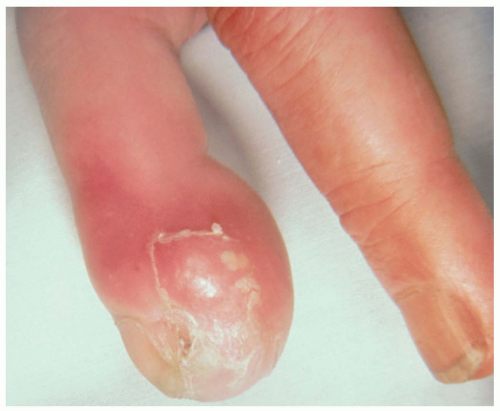
Patients with felons present complaining of throbbing pain and swelling in the fingertip. Pain usually develops over a few days. On examination, there is a reddened, warm fingertip with fluctuance evident.
Pathophysiology
A felon is an abscess of the pulp space of the distal phalanx. Most felon infections are caused by introduction of Staphylococcus aureus into the pulp space through microtears in the skin. Infections with streptococci and gram-negative organisms also occur in the pulp space. Septations in the pulp usually isolate the infection to the pulp space.1 Splinters or other foreign bodies may precipitate infection, but usually felons arise without any identifiable source of trauma.
Diagnosis
Diagnosis of felon is made on the basis of history and classic physical findings. Cultures may be useful if there is evidence of underlying osteomyelitis. Plain radiographs are reserved for those cases in which a foreign body or osteomyelitis is suspected.1
Clinical Complications
Osteomyelitis of the distal phalanx may occur if the infection in the pulp space is left untreated. Skin, soft tissue, and nerve necrosis may also occur if the infection is not promptly treated.1
Management
Treatment of felon involves incision and drainage of the infected pulp space. A digital block is used for anesthesia. A high lateral incision is made, starting just distal to the distal fold of the nail and continuing parallel to the nail plate. Curved hemostats are then used to gently break up loculations. This incision preserves the neurovascular bundle. An alternative approach is through a longitudinal incision in the volar aspect of the fingertip (the fatpad), ending distal to the distal interphalangeal (DIP) joint to avoid inadvertent injury to the flexor sheath or flexor digitorum profundus insertion. The drawback of this incision is the potential for a painful fingertip scar. The wound should be thoroughly irrigated and then packed if necessary. The patient should be prescribed oral antistaphylococcal antibiotics for 1 week and seen again in 2 days for follow-up.1
REFERENCES
1. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
15-5 Digit Amputation
Mark Silverberg
Clinical Presentation
Patients with digit amputation (DA) present after industrial or agricultural accidents, high-speed trauma events, or assaults with sharp weapons.
Pathophysiology
A complete DA denotes a finger or toe that is completely severed from the body, whereas an incomplete DA implies that the digit is still partially attached by a pedicle but the arterial circulation has been disrupted.1 Amputation is better tolerated in children than in adults.1 Guillotine-type, clean amputations are best suited for reimplantation, but most DAs result from crush or avulsion mechanisms.1 The viability of an amputated digit may be maintained for up to 6 hours of warm ischemia and more than 12 hours of cold ischemia.1
Diagnosis
DAs can be classified according to the anatomic location of the amputation. Zone I DAs occur distal to the bony structures of the digit. Injuries classified as zone II are located distal to the lunula of the nail bed and are complicated by bone exposure. Zone III injuries involve the nail matrix and usually result in loss of the entire nail.2
Clinical Complications
Complications include reimplantation failure, permanent loss of function, infection, osteomyelitis, reflex sympathetic dystrophy, and permanent sensory changes that include paresthesias, hyperesthesia, or a sensation of coldness.2
Management
The patient should be assessed for other injuries, and the digit should be wrapped in gauze moistened with saline and placed inside a plastic bag. That bag should then be placed on ice. The digit must not be allowed to freeze.1 The patient should be transferred to a reimplantation center if the hospital does not have that service.1,2 Attempts at digital reattachment should take place at a specialized reimplantation center.1 The order of reimplantation is bone first, followed by tendons, arteries, veins, and then nerves.2 The entire site should be wrapped in a compression dressing, and skin grafts may or may not be used. Tetanus prophylaxis should be checked and updated if necessary, and parenteral antibiotics should be administered.1
REFERENCES
1. Michalko KB, Bentz ML. Digital replantation in children. Crit Care Med 2002;30[11 Suppl]:S444-S447.
2. Jackson EA. The V-Y plasty in the treatment of fingertip amputations. Am Fam Physician 2001;64:455-458.
15-6 Nail Avulsion
James Nelson
Colleen Campbell
Clinical Presentation
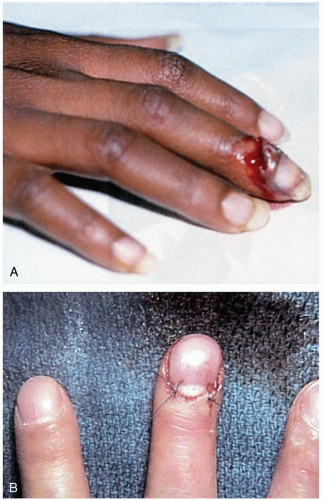
Patients with nail avulsion present with pain and bleeding at the nail bed after trauma. A laceration of the nail bed often accompanies avulsion injuries. In partial avulsions, an underlying nail-bed injury should be suspected if there is a subungual hematoma occupying more than 25% of the nail-bed area.1
Pathophysiology
Simple separation of the nail from its nail bed does not disrupt the underlying germinative centers. The germinative matrix forms the new nail proximally to distally, at a rate of 1 mm/day, so that it often takes 6 months for complete nail formation after injury.
Diagnosis
Nail avulsion is a clinical diagnosis. Radiographs should be obtained if a tuft fracture is suspected.1
Clinical Complications
Failure to adequately repair the nail bed can cause irregularities in the new nail. There may be permanent loss of the nail if the injury is severe.1
Management
The most effective treatments have not been completely elucidated. Nail-bed lacerations should be thoroughly cleansed and irrigated, especially if there is an underlying tuft fracture. If laceration of the nail bed is suspected, the nail should be removed for further inspection and repair. Repair of the nail bed can be done with the use of 6-0 or 7-0 absorbable suture.1 Some recommend using the nail, if it is intact, as a splint for 2 to 3 weeks. Alternatively, the nail bed may be covered with a nonadhesive dressing shaped like a nail. Antibiotics directed at skin organisms are used prophylactically if an open tuft fracture is present.1
REFERENCES
1. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
15-7 Nail Bed Injury
Tyler Vadeboncoeur
Clinical Presentation
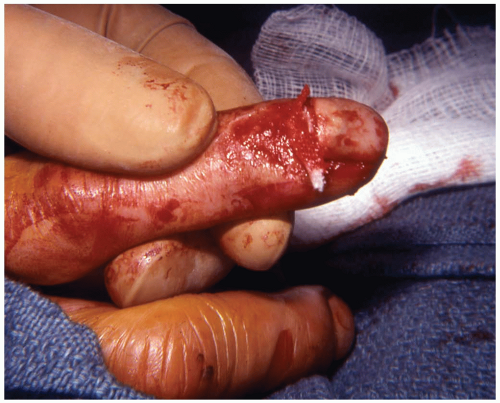
Patients with nail bed lacerations present after high-force crush injuries or lacerations. A grossly deformed nail, with or without a nail-bed laceration, is evident.1
Pathophysiology
Crush injuries range from subungual hematomas to amputation. An additional mechanism of injury is catching and ripping off the nail plate, with resultant laceration or avulsion of the nail bed. Approximately 50% of injuries involve fracture to the distal phalanx.1
Diagnosis
Simple injuries are diagnosed by history, examination, and radiography to assess the presence of fracture or foreign body. Complex injuries may require digital block followed by cleansing and a close evaluation with loupe magnification.1,2,3
Clinical Complications
Nail-plate deformity affecting permanent nail growth is a common complication.2
Management
Subungual hematomas with partially adherent nail plates that are still aligned in the nail fold may be treated with trephination, regardless of the size of the hematoma.3 Nail-bed lacerations may be repaired after a digital block and exsanguination of the finger. If the nail plate is still attached and is still within the nail fold, it generally does not need to be removed. If the nail is displaced, it should be removed and the nail bed further examined. Repair of lacerations is performed with 6-0 or 7-0 absorbable suture under loupe magnification. The nail may then be sutured in place or taped as a temporary splint to protect the underlying nail bed and maintain the fold for new nail growth. If the nail is not available, a single thickness of nonadherent gauze may be used in its place.1 Complex injuries in which the nail bed cannot be accurately repaired, or in which nail-bed fragments are lost, should be referred to a hand surgeon. Tuft fractures do not alter the management of nail-bed injuries, but more proximally displaced fractures might require reduction.1 Tetanus prophylaxis should be administered as needed.1,2,3
REFERENCES
1. Brown RE. Acute nail bed injuries. Hand Clin 2002;18:561-575.
2. Hart RG, Kleinhart HE. Fingertip and nail bed injuries. Emerg Med Clin North Am 1993;11:755-765.
3. Roser SE, Gellman H. Comparison of nail bed repair versus nail trephination for subungual hematomas in children. J Hand Surg Am 1999;24:1166-1170.
15-8 Subungual Hematoma
Colleen Campbell
Clinical Presentation
Patients with subungual hematomas present with throbbing pain at the fingertip and purple-red discoloration underlying the nail after trauma to the hand.
Pathophysiology
Diagnosis
Diagnosis of subungual hematoma is made on the basis of history, physical examination, and the finding of blood visible under the nail. Radiographs of the finger (anteroposterior, lateral, and oblique) are necessary to evaluate possible underlying fractures of the distal phalanx.1
Clinical Complications
Loss of the overlying nail is common after a large subungual hematoma. Subungual abscess or infection may also result from retained blood.
Management
Subungual hematomas involving more than 25% of the nail, as well as those associated with substantial pain, require trephination. This may be accomplished with the use of an electrocautery tip, a heated paperclip, or a sterile large-bore needle.1 The needle is carefully twisted through the overlying nail until blood is released from under the nail through the hole. If the hematoma involves more than half of the nail bed, it may be necessary to remove the nail to repair the nail bed. Digital anesthesia is administered, followed by nail bed removal. The nail may be replaced and secured with a suture, or the nail bed may be covered with petrolatumimpregnated gauze.1 A padded aluminum splint is then applied for added protection of the fingertip.
REFERENCES
1. Wang QC, Johnson BA. Fingertip injuries. Am Fam Physician 2001; 63:1961-1966.
2. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
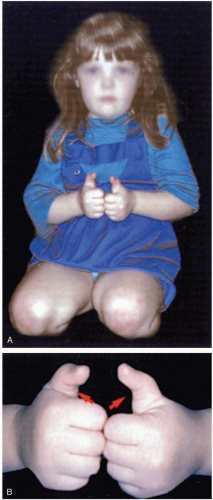
15-9 Clenched-Fist Injury (“Fight Bite”)
Michael Greenberg
Clinical Presentation
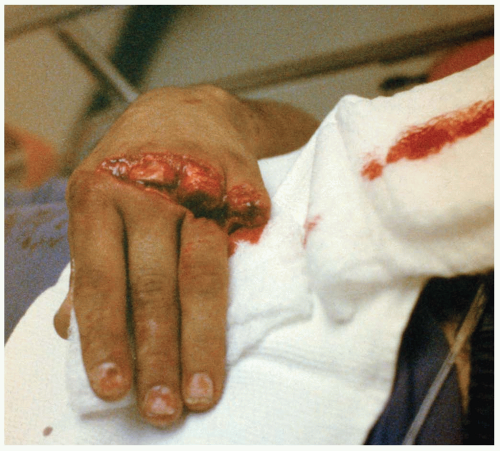
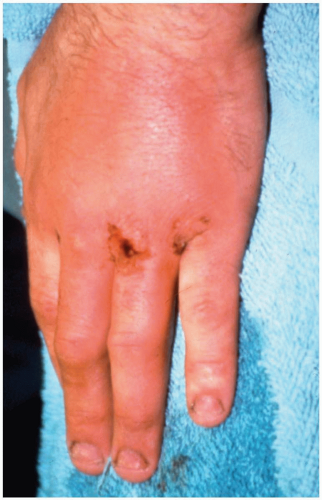
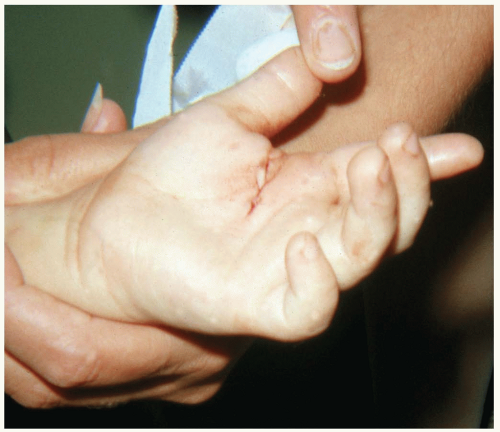
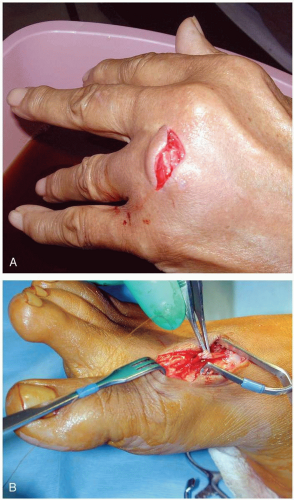
Some patients with clenched-fist injuries present immediately after the injury with swelling and pain around a laceration over the dorsum of the third through fifth metacarpal joints. Other patients present 1 to 3 days after the initial injury with swelling, redness, severe pain, and decreased range of motion.1
Pathophysiology
Clenched-fist injuries result when a person strikes another individual in the mouth region with the fist. If the soft tissue injury includes a break in the integrity of the skin on the dorsum of the hand, infection can complicate the original injury.1 Fist-to-mouth contact may be the most common cause of human bite wounds. These “fight bite” wounds have the highest incidence of complications of any closed-fist injury, as well as any type of bite wound.1
Diagnosis
A high index of suspicion for “fight bite” is essential in any injury involving lacerations, abrasions, or bruising over the metacarpophalangeal (MCP) joints.1 The wound must be thoroughly explored to rule out joint capsule violation and a retained foreign body, such as a tooth fragment or piece of jewelry. Exploration must be done throughout the range of motion, because the injury may be noted only when the hand is flexed, as in a fist. The wound should be copiously irrigated. If the joint space was violated, it should be thoroughly irrigated as well. The wound should be left open. No primary closure should be attempted, because such an approach increases the possibility of infection.1
Management
After careful examination, these wounds should be infiltrated with local anesthetic, soaked and vigorously scrubbed, and then carefully explored. Patients should receive a parenteral broad-spectrum antibiotic in the emergency department, as well as continued outpatient oral antimicrobial agents.1 Early follow-up (24 to 48 hours) with a physician skilled in the management of these injuries is encouraged. If the patient appears noncompliant, consideration should be made for initial inpatient care. Early range of motion exercise is initiated, and the wound is allowed to heal-in secondarily or closed as a delayed primary closure.1
REFERENCES
1. Perron AD, Miller MD, Brady WJ. Orthopedic pitfalls in the ED: fight bite. Am J Emerg Med 2002;20:114-117.
15-10 Flexor Tendon Laceration
Michael Greenberg
Clinical Presentation
Patients with flexor tendon lacerations (FTLs) present after lacerations or punctures of the volar surface of one or more digits. Patients may complain of pain and inability to flex all or part of the digits distal to the injury. Other patients may be unaware of their inability to flex the digits until function is fully tested by the physician.
Pathophysiology
FTLs may be partial or complete, depending on the nature of the injury and the depth of the wound. Injury to the tissue around the tendon can result in decreased nutrition to the tendon due to inadequate blood flow, decreased synovial fluid, or other factors.1 Many factors can influence effective healing and result in weak repairs, postrepair adhesions, or postrepair scarring. All of these issues make flexor tendon repair “one of the most challenging problems in orthopedic surgery.”1 The flexor tendons are weakest at approximately 21 days after repair.
Diagnosis
Accurate diagnosis hinges on a complete history and careful physical examination. The emergency physician is usually the first physician to see the patient who has sustained an FTL and must take caution never to miss the diagnosis. Any laceration involving the volar surface of the digits or hand must be considered suspect for flexor tendon injury. The examination must include placing the injured digits through both passive and active range of motion. In many cases, accessory muscles provide functionality, creating the appearance that no tendon injury has occurred. However, the diagnosis will not be missed if the physician uses careful sterile, bloodless, extension of the wound with a scalpel to allow full visualization. The examination must also include a careful assessment of the neurovascular aspects of the injured areas.
Clinical Complications
Complications include missing the diagnosis of FTL, infection, posttreatment digital stiffness, functional disability, and peritendinous adhesions.
Management
Surgical repair usually is required. Therefore, the primary role for the emergency physician is accurate diagnosis, wound cleansing, and prompt referral. Antibiotics should be given in consultation with the consulting surgeon. Tetanus immunization should be updated as needed.
REFERENCES
1. Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am 2003;85:539-550.
15-11 Extensor Tendon Laceration
Michael Greenberg
Clinical Presentation
Patients with extensor tendon lacerations (ETLs) present with inability to normally extend one or more digits or the hand at the wrist.
Pathophysiology
ETLs may result from punctures or lacerations to the dorsal surfaces of the hand, wrist, or digits.
Diagnosis
The diagnosis may be made or suspected by having the patient extend the affected digits or the hand at the wrist, both passively and against gentle resistance. With an open wound, it may be necessary to widely extend the wound, using a sterile scalpel and sterile technique, to allow maximal visualization of the tendon and its sheath. Direct inspection should then be performed under both passive and resistive full range of motion. The temporary use of a blood-pressure cuff may facilitate inspection in this manner by creating a relatively bloodless field.
Clinical Complications
Complications include missing the diagnosis of ETL, infection, chronic pain, and disability.
Management
In some cases, partial laceration of one or more extensor tendons located proximal to the metacarpophalangeal (MCP) joint does not require surgical repair. These injuries are often treated expectantly, with immobilization and early rehabilitation. Many lacerations that extend through less than 30% of the tendon width may be treated in this fashion, but partial lacerations of the extensor tendons at, or distal to, the MCP joint uniformly require suture repair. The strength of a tendon repair, once healing has occurred, depends on the number and size of sutures crossing the laceration site. The most efficient repair technique involves the use of running horizontal mattress sutures. Simple ETL repair may be accomplished in the emergency department but should be performed by a physician with training and experience in the surgical treatment of this injury.
REFERENCES
1. Russell RC, Jones M, Grobbelaar A. Extensor tendon repair: mobilise or splint? Chir Main 2003;22:19-23.
2. Calabro JJ, Hoidal CR, Susini LM. Extensor tendon repair in the emergency department. J Emerg Med 1986;4:217-225.
15-12 Trigger Finger
Michael Greenberg
Clinical Presentation
The age distribution for trigger finger is bimodal: before 6 years of age, and after 40 years of age.1 Patients present with difficulty in flexing or extending the digit, and pain may be present. A snapping sensation is reported when the finger is extended.
Pathophysiology
Trigger finger is characterized by snapping or locking of the thumb or fingers. This condition is also known as flexor tendon entrapment of the digits.1 Most cases of trigger finger are related to thickening of the digit’s first annular pulley, but other causes include trauma, inflammatory response, diabetes, or heredity.2 The condition generally occurs in the palm at the metacarpophalangeal (MCP) joint. Triggering results from a disproportion between the diameter of the flexor tendons and the diameter of the fibro-osseous canal. Trigger finger may occur rarely at the level of the other annular pulleys, the carpal tunnel, or the dorsal compartments.1
Diagnosis
Palpation during motion may reveal a snapping sensation. A nodule may also be felt on the palmar side of the MCP joint. Radiographic evaluation is not helpful in diagnosing this condition.1
Clinical Complications
Trigger finger is usually a minor condition for most patients, who report it to be only an annoyance. However, depending on the patient’s occupation, trigger finger can be disabling (e.g., musicians, technicians). Trigger finger can be chronic and progressive, with the digit eventually becoming locked in flexion.1
Management
Corticosteroid injection may be used to treat trigger finger, especially in patients with rheumatoid arthritis. It may be used in conjunction with a 3-week course of splinting that extends the MCP joint while allowing the interphalangeal joints to remain mobile. Surgical treatment for severe trigger finger may become necessary.1,3
REFERENCES
1. Moore J. Flexor tendon entrapment of the digits (trigger finger and trigger thumb). J Occup Environ Med 2000;42:526-545.
2. Gaffield J, Mackay D. A-3 pulley trigger finger. Ann Plastic Surg 2001;46:352-353.
3. Bain G, Wallwork N. Percutaneous A1 pulley release: a clinical study. J Bone Joint Surg Br 1998;80:131.
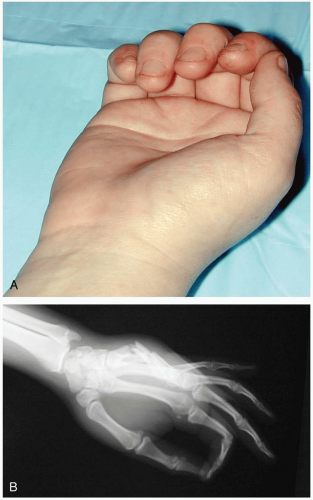
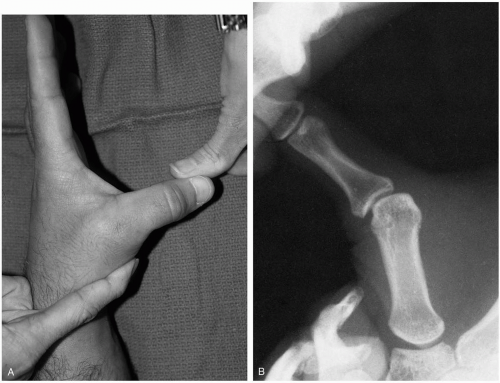
15-13 Finger Dislocations
Colleen Campbell
Clinical Presentation
Patients with finger dislocations present with pain in the affected finger.
Pathophysiology
Dislocations often occur as a result of injuries sustained during ball sports. Dislocations occur most frequently at the proximal interphalangeal (PIP) joint, followed by the metacarpophalangeal (MCP) joint and, rarely, the distal interphalangeal (DIP) joint.1 Most dislocations are dorsal, but they can be lateral or volar. Dorsal dislocations are caused by longitudinal compression during a hyperextension injury.1 Full dislocation occurs as a result of bilateral splitting of the collateral ligaments in combination with avulsion of the volar plate.1 There are three different types of dislocations. Type I is a hyperextension injury that results in a partial slip of the collateral ligament, with avulsion of the volar plate of the middle phalanx. Type II is a full dislocation and volar-plate avulsion, and type III is a fracture-dislocation.
Diagnosis
Dislocation usually is suspected on the basis of physical examination findings. Radiographs of the finger, including anteroposterior, lateral, and oblique views, are necessary to confirm dislocation and to evaluate for associated fracture. Tenderness and obvious deformity of the affected joint are present on examination. The joint may appear hyperextended.
Clinical Complications
Joint stiffness is common after dislocations, which is why early movement of the joint through its range of motion is advocated. Chronic instability may follow if the injury is not properly treated.
Management
Reduction usually can be achieved with gentle traction distal to the dislocation. For MCP dislocations, distally directed pressure should be applied to the base of the proximal phalanx, with simultaneous gentle distraction of the proximal phalanx.2 A digital block using lidocaine may be useful before reduction. Type I and II dislocations require dynamic splinting (buddy tape) for 3 weeks, with range-of-motion exercises afterward. Treatment of type III injuries depends on the stability of the joint in flexion. If the joint is stable, treatment with dynamic splinting for 6 weeks is often successful. Joints are more prone to instability if the fracture involves more than 40% of the articular surface.1 In these cases, extension block splinting, joint arthroplasty, or rigid fixation with Kirschner wiring may be necessary.1
REFERENCES
1. Palmer RE. Joint injuries of the hand in athletes. Clin Sports Med 1998;17:513-531.
2. Young CC, Raasch WG. Dislocations: diagnosis and treatment. Clin Fam Pract 2000;2:613-635.
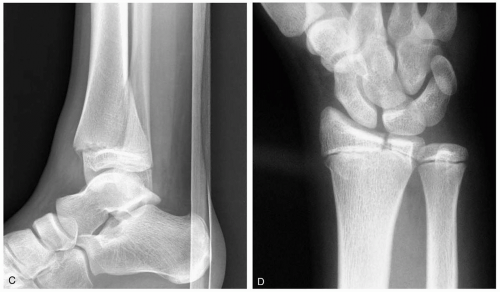
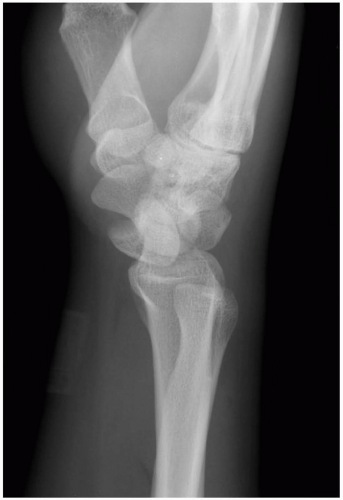
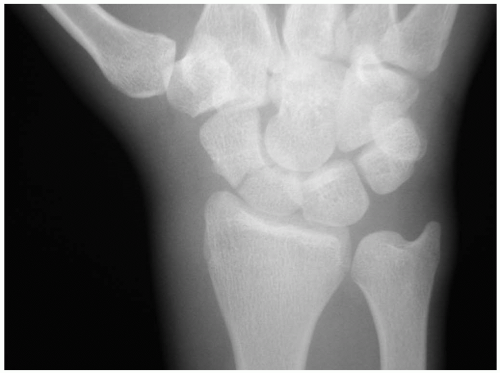
15-14 Lunate Dislocation
Catherine Pelletier
Clinical Presentation
Patients with lunate dislocations present with swelling of the dorsum of the wrist and pain associated with range of motion. Symptoms consistent with neuropathy in the distribution of the median nerve may also be present.
Pathophysiology
Lunate dislocations usually are associated with a mechanism of injury that produces forceful dorsiflexion of the wrist, such as those associated with falls onto an outstretched hand or motor vehicle crashes.1
Diagnosis
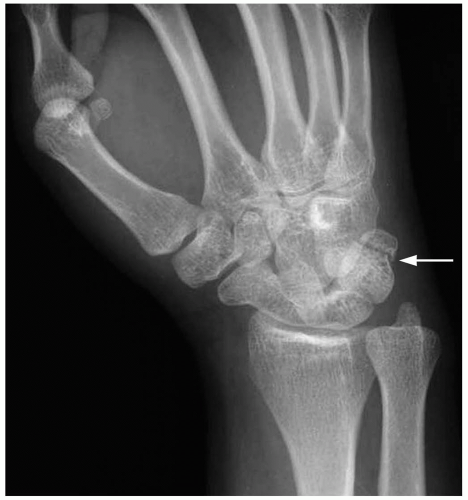
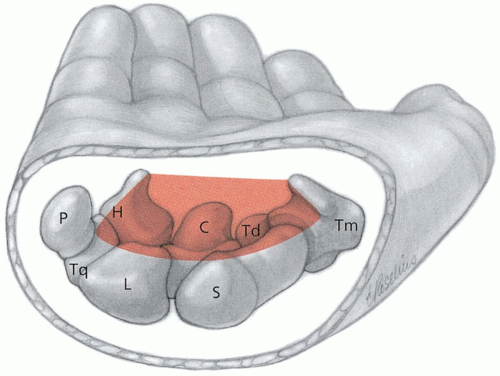
The diagnosis of lunate dislocation is based on clinical examination in conjunction with posteroanterior (PA) and lateral wrist radiographs. On the PA view of the wrist, the volar dislocation of the lunate appears as a wedged-shaped “piece of pie,” as opposed to its usual quadrangle appearance. As the lunate tips toward the palmar aspect of the wrist, the lateral wrist radiographic appearance is that of a “spilled teacup.” The capitate slips behind the lunate and may even move proximally, toward the radius. Care must be taken when reviewing wrist radiographs, because up to 20% of all carpal pathologic conditions are erroneously interpreted as being normal.1,2
Clinical Complications
Complications include chronic pain and disability, scapholunate collapse secondary to avascular necrosis, and damage to the median nerve.
Management
Orthopedic consultation from the emergency department is the critical step in management of lunate dislocations, because patients require admission with urgent open reduction and internal fixation for definitive care of this injury.1,3,4 Careful splinting is required while awaiting the arrival of the orthopedist.
REFERENCES
1. Yaghoubian R, Goebel F, Musgrave DS, Sotereanos DG. Diagnosis and management of acute fracture-dislocation of the carpus. Orthop Clin North Am 2001;32:295-305.
2. Perron AD, Brady WJ, Keats TE, Hersh RE. Orthopedic pitfalls in the ED: lunate and perilunate injuries. Am J Emerg Med 2001;19:157-162.
3. Cooney WP, Bussey R, Dobyns JH, Linscheid RL. Difficult wrist fractures: perilunate fracture-dislocations of the wrist. Clin Orthop 1987;(214):136-147.
4. Ferenz CC. Acute perilunate dislocations. Orthop Rev 1986;15:213-217.
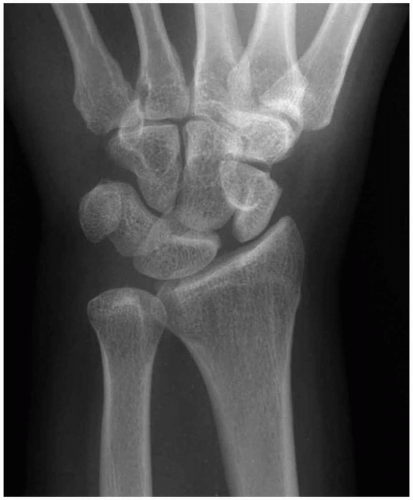
15-15 Perilunate Dislocations
Catherine Pelletier
Clinical Presentation
Patients with perilunate dislocations present to the emergency department with wrist pain, swelling over the dorsum of the wrist, and limited range of motion secondary to pain. Physical deformity of the wrist may also be present.
Pathophysiology
Perilunate dislocations are a specific type of carpal instability representing 1.5% of all carpal fractures.1 Perilunate dislocations are associated most commonly with a fall onto an outstretched hand, although it can be caused by any mechanism of injury that produces forceful dorsiflexion of the wrist. A high level of clinical suspicion must be maintained in the setting of these types of injuries, because up to 20% of radiographs of carpal pathologic conditions are initially misinterpreted as normal.1
Diagnosis
Diagnosis is made via posteroanterior (PA) and lateral wrist radiographs. On a lateral wrist radiograph, the capitate no longer forms the fourth “C” in a linear row formed by the distal radius, the proximal aspect of the lunate, the distal aspect of the lunate, and the proximal aspect of the capitate. This obscures the separation between the proximal and distal rows of the carpal bones on the PA view. This injury is often associated with fracture or subluxation of the scaphoid. The median nerve is also commonly injured because of its proximity to the carpal bones.1,2
Clinical Complications
Complications include median-nerve injury, posttraumatic arthritis, avascular necrosis of the lunate and/or scaphoid, and chronic pain.
Management
Early radiographic recognition of these injuries is necessary. Closed reduction and long-arm splint application was once the standard of care and may still be attempted, although better outcomes result after open reduction and internal fixation, especially in high-risk, noncompliant populations.2,3,4,5
REFERENCES
1. Perron AD, Brady WJ, Keats TE, Hersh RE. Orthopedic pitfalls in the ED: lunate and perilunate injuries. Am J Emerg Med 2001;19:157-162.
2. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am 1993;18:768-779.
3. Cooney WP, Bussey R, Dobyns JH, Linscheid RL. Difficult wrist fractures: perilunate fracture-dislocations of the wrist. Clin Orthop 1987;(214):136-147.
4. Ferenz CC. Acute perilunate dislocations. Orthop Rev 1986;15:213-217.
5. Yaghoubian R, Goebel F, Musgrave DS, Sotereanos DG. Diagnosis and management of acute fracture-dislocation of the carpus. Orthop Clin North Am 2001;32:295-305.
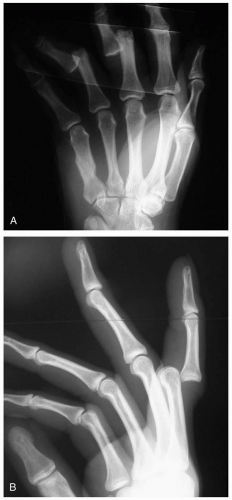
15-16 Phalanx Fractures (Hand)
Colleen Campbell
Clinical Presentation
Patients with phalanx fractures present after trauma to the hand with pain and swelling of the fingers or thumb. Deformity may be present as well. Rotational deformity is usually evident on examination of flexed fingers in the palm. All fingers should be aligned and pointed toward the scaphoid tubercle.1
Pathophysiology
Phalanx fractures occur as a result of crush or shearing forces. The distal phalanx is injured most often.2 Fractures are usually transverse, longitudinal, or comminuted.3 Transverse fracture patterns are the most common, because most injuries are the result of a direct blow.1 Proximal phalanx fractures often have a degree of volar angulation because of the action of the interosseous muscles. Sports-related injuries are the most common cause of phalanx fractures in teenagers and young adults.1
Diagnosis
Bony point tenderness is evident on examination. Diagnosis is confirmed by plain radiography, including anteroposterior, lateral, and oblique views of the finger. If multiple fingers are injured, a splayed view of the hand may be necessary. Brewerton views can be used to further visualize the base of the proximal phalanx.1
Clinical Complications
Malunion is the most common complication of phalanx fractures. Other complications include posttraumatic arthritis, decreased range of motion, and deformity of the finger.2
Management
Fractures of the distal phalanx may be treated with a hairpin splint that extends to the middle phalanx. Kwires or plates may be necessary to fix severely angulated, unstable, or displaced fragments.1 Fractures with associated nail bed injuries are considered to be open. Treatment with antistaphylococcal antibiotics is controversial.3 Stable, nondisplaced proximal and middle phalanx fractures are treated with “buddy” taping.2 Other fractures may be treated after reduction with a radial gutter splint (index and long fingers) or an ulnar gutter splint (small and ring fingers) in a position of function. The wrist is placed in 30 degrees of extension, the metacarpophalangeal (MCP) joints in 90 degrees of flexion, and the interphalangeal (IP) joints in extension.1 Orthopedic referral is indicated for all unstable, angulated, and intraarticular fractures.3
REFERENCES
1. Capo JT, Hastings H II. Metacarpal and phalangeal fractures in athletes. Clin Sports Med 1998;17:491-511.
2. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
3. Wang QC, Johnson BA. Fingertip injuries. Am Fam Physician 2001; 63:1961-1966.
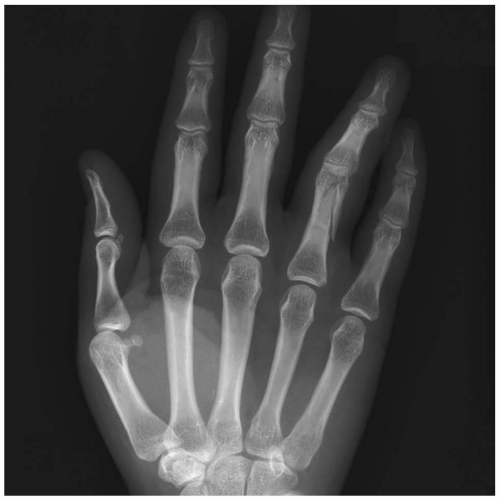
15-17 Metacarpal Fractures
Robert Hendrickson
Clinical Presentation
Patients with metacarpal (MC) fractures present as a result of blunt trauma sustained to the hand, with swelling, pain, and tenderness in the area of injury.
Pathophysiology
MC fractures can occur at various locations in the MC bones, including the base, shaft, neck, and head of the bone. Force may be transferred to the MC bones by a direct blow to the hand, either perpendicularly, longitudinally, or rotationally.
Diagnosis
The diagnosis may be suspected by recognizing swelling, pain, and tenderness on examination and should be confirmed with radiography. The integrity of the neurovascular aspects of the affected areas should be evaluated and the hand should be examined for rotational deformities. Fractures usually are visible on radiography. A Bennett’s fracture is an intraarticular fracture/dislocation of the thumb metacarpal. Rolando’s fractures are Y-shaped, comminuted Bennett’s fractures.
Clinical Complications
Complications include chronic pain, arthritis, open fracture, and significant occupational and functional disability.
Management
Open fractures of the MC bones require immediate sterile irrigation and débridement, often best done in the operating theater.1 Treatment decisions regarding reduction, splinting, and consultation depend primarily on the location of the fracture.
Fractures of the head of the MC typically occur after a direct blow or crush injury. These fractures should be splinted with a gutter splint, and the patient should be referred to a hand surgeon, because the outcome for these injuries tends to be poor, regardless of treatment. Fractures of the metacarpal neck should be treated with closed reduction if there is angulation greater than 15, 15, 35, or 45 degrees in the second, third, fourth, or fifth metacarpophalangeal (MCP), respectively. The injury should be splinted in a gutter splint from the elbow up to, but not including, the proximal interphalangeal joint, and the patient should be referred to a hand surgeon. Fractures of the MC shaft require closed reduction if there is any angulation of the second or third MC, or if the angulation of the fourth or fifth MC is greater than 10 or 20 degrees. The gutter splint should extend up to, but should not include, the MCP joint, and the patient should be referred to a hand surgeon. Patients with fractures of the second through fifth MC bases require a volar splint and referral to a hand surgeon. Fractures of the first MC may require reduction if they are either intraarticular or extraarticular with greater than 20% to 30% angulation. After reduction, a thumb spica splint may be applied, and the patient is referred to a hand surgeon for open reduction and internal fixation.1
REFERENCES
1. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
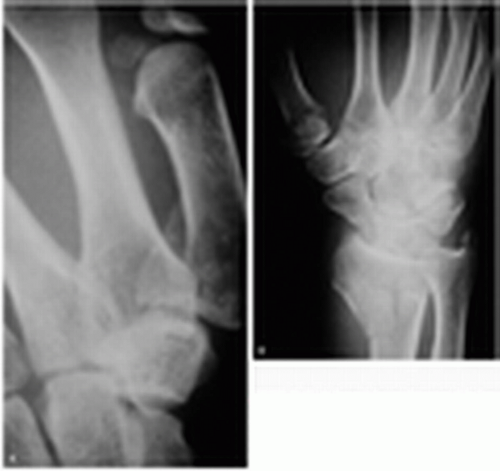
15-18 Scaphoid Fracture
Mark Silverberg
Clinical Presentation
Patients with scaphoid fractures present with moderate to severe wrist pain, usually after a fall on an outstretched hand. Swelling and ecchymosis may also be present, and wrist motion is commonly limited.
Pathophysiology
The scaphoid is the most commonly fractured carpal bone, accounting for 70% to 80% of all carpal fractures.1 The most common mechanism of injury is a fall onto an outstretched hand.
Diagnosis
Physical examination reveals exquisite tenderness in the anatomic snuff box and pain when the thumb is axially loaded. In addition, pain with resisted supination or pronation is typical.1 Normal wrist radiographs are usual. However, a scaphoid view showing the scaphoid on its long axis should also be obtained. In 85% of scaphoid fractures, the navicular fat stripe (a radiolucent line parallel to the radial surface of the scaphoid bone on the anteroposterior projection) is laterally displaced or completely obliterated.1 Negative radiographic findings do not necessarily rule out a scaphoid fracture. If the patient appears to have a fracture clinically and the radiographs are negative, the patient should be placed in a short-arm thumb spica cast, and the radiographs should be repeated in 10 to 14 days. A bone scan or magnetic resonance imaging scan performed 72 hours after the injury may reveal scaphoid fractures that do not appear on plain films.1
Clinical Complications
Because of the distal-to-proximal blood supply of the scaphoid bone, fractures through the wrist and proximal region are prone to ischemia. This increases the risk for prolonged healing and nonunion, as well as avascular necrosis resulting in chronic wrist pain.1
Management
Nondisplaced fractures of the distal third or scaphoid tubercle usually heal well and may be splinted, with the patient sent for follow-up with an orthopedic surgeon. These fractures usually are placed in a short-arm thumb spica cast for 10 to 12 weeks. Some orthopedists obtain follow-up radiographs every 2 to 3 weeks until radiographic healing is demonstrated.1 Rapid orthopedic consultation should be obtained for proximal fractures, comminuted fractures, and fractures displaced more than 2 mm, because of the high risk of complications. These patients usually require a long-arm thumb spica and should be monitored closely by an orthopedist.1
REFERENCES
1. Paras RD. Upper extremity fractures. Clin Fam Pract 2000;2:637-659.
15-19 Scapholunate Dislocation
Tyler Vadeboncoeur
Clinical Presentation
Pathophysiology
Scapholunate dislocations are the most common ligamentous injury of the wrist; they occur when the scapholunate interosseous ligament and the long radiolunate ligaments tear. This causes the scaphoid to be displaced to a more vertical position relative to the lunate.1,2
Diagnosis
The diagnosis is made radiographically. The “signet ring” sign results with volar rotation of the scaphoid. The radiographic criteria for dissociation include a scapholunate angle greater than 60 degrees; a scapholunate space wider than 2 mm (the Terry Thomas sign); and the scaphoid ring sign, either singly or in any combination. In the case of suspected dissociation with normal-appearing routine radiographs, additional stress views should be taken with a clenched fist in ulnar deviation, which accentuates the widening of the scapholunate joint.2 Acute scapholunate dislocations may be asymptomatic or mildly symptomatic.
Clinical Complications
Management
Acute scapholunate dislocations require hand surgery consultation in the emergency department. The injury should be splinted in a radial gutter splint or posterior mold. Subacute or chronic injuries may be casted or splinted, and they also require hand surgery referral.1 These injuries may require radiographically guided reduction and pinning and usually require open reduction with internal fixation for ligamentous repair.1
REFERENCES
1. Lee JS, Gaala A, Shaw R, Harris JH Jr. Signs of acute carpal instability associated with distal radius fracture. Emerg Radiol 1995;2:77-83.
2. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am 1980;5:226-241.
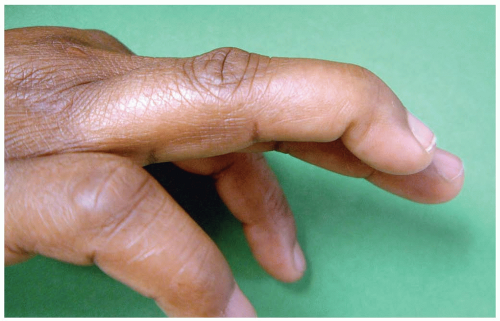
15-20 Swan Neck Deformity
Colleen Campbell
Clinical Presentation
Patients with swan neck deformity (SND) present with only the flexion deformity of the distal interphalangeal (DIP) joint after injury. On examination, the acutely injured patient has pain at the DIP joint with swelling, tenderness, and inability to actively extend the DIP of the affected digit. Over time, patients develop hyperextension at the proximal interphalangeal (PIP) joint. SND usually is not painful.
Pathophysiology
SND occurs as a progression of mallet finger, when there is a flexion deformity of the DIP combined with a hyperextension of the PIP. Mallet finger occurs when there is forced flexion of the extended DIP.1 Mallet finger, the most common finger injury in athletes, is caused by blunt trauma. SND occurs when a mallet finger goes untreated; the flexion deformity of the DIP causes chronic strain and eventually failure of the lateral bands. The PIP is hyperextended when the lateral bands are displaced dorsally and proximally.1
Diagnosis
Diagnosis of SND is made on the basis of physical examination. Anteroposterior, lateral, and oblique radiographs of the finger are necessary to reveal an associated fracture.
Clinical Complications
Complications of SND include post-traumatic arthritis and chronic deformity or chronic subluxation and associated chronic disability.
Management
Treatment of mallet fingers initially involves splinting the DIP in extension for 6 to 8 weeks.1 For SND, prompt orthopedic referral is warranted, given the chronic nature of the condition.
REFERENCES
1. Wang QC, Johnson BA. Fingertip injuries. Am Fam Physician 2001;63:1961-1966.
2. Palmer RE. Joint injuries of the hand in athletes. Clin Sports Med 1998;17:513-531.
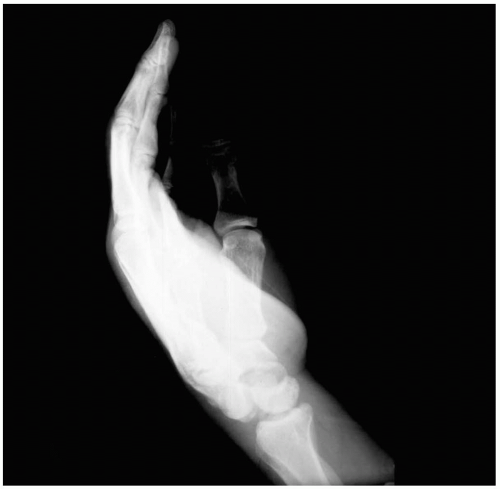
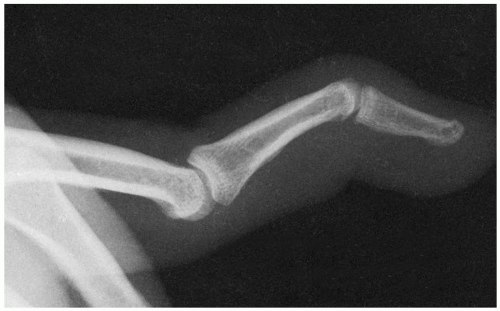
15-21 Mallet Finger
Michael Greenberg
Clinical Presentation
Mallet finger injuries usually are not excessively painful, and therefore presentation may be delayed. Patients may have ecchymosis over the dorsum of the distal interphalangeal (DIP) joint. There may not be any tenderness, swelling, or discoloration. However, all patients demonstrate an extensor lag at the DIP joint.1
Pathophysiology
Mallet finger, also known as “baseball finger” or “drop finger,” is a flexion deformity that is caused by the detachment of the extensor tendon from the base of the distal phalanx.2 Mallet injuries are classified into four types. Type I is closed, with or without associated avulsion fracture. Type II involves a laceration proximal to the DIP joint with loss of tendon continuity. Type III mallet fingers are deep abrasion injuries with the loss of skin, subcutaneous cover, and tendon substance. Type IV mallet fingers can involve either pediatric transepiphyseal plate fractures or fractures of the articular surface.3 The mechanism of injury usually involves direct trauma that forces an extended finger into flexion. The direct blow could also occur to the dorsum of the finger.1 The extensor tendon is ruptured, and there is often an associated bony avulsion.1,3
Diagnosis
On clinical evaluation, patients exhibit an extensor lag at the DIP joint. This lag may not develop for several days after injury, so patients may present to the emergency department late in the course of events. The magnitude of the lag can vary according to the severity of the mallet injury. Diagnosis is confirmed by plain radiography.1
Clinical Complications
Patients may believe that mallet injuries are simple problems that have minor complications. If the injury is left untreated, however, a “swan neck” deformity may develop in the digit due to hyperextensive compensation at the proximal interphalangeal (PIP) joint. Untreated mallet injuries can become chronically painful.2
Management
Surgical treatment reportedly has a higher morbidity than conservative, nonsurgical treatment.2 Splinting alone may be satisfactory in most cases. Type I injuries can be treated with continuous DIP joint splinting for up to 8 weeks. Type II injuries may be corrected with suturing, followed by splinting. Type III mallet fingers require immediate soft-tissue coverage and grafting reconstruction. Type IV injuries that involve transepiphyseal plate fractures may be treated with closed reduction.3 Patients must understand that if they remove the splinting before clinical follow-up, they cannot let the DIP joint fall into flexion.1
REFERENCES
1. Perron A, Brady W, Keats T, Hersh R. Orthopedic pitfalls in the emergency department: closed tendon injuries of the hand. Am J Emerg Med 2001;19:76-80.
2. Okafor B, Mbubaegbu C, Munshi I, Williams D. Mallet deformity of the finger: five-year follow-up of conservative treatment. J Bone Joint Surg Br 1997;79:544-547.
3. Rockwell W, Butler P, Byrne B. Extensor tendon: anatomy, injury, and reconstruction. Plastic Reconstruct Surg 2000;106:1592-1603.
15-22 Gamekeeper’s Thumb
Grant Wei
Clinical Presentation
Pathophysiology
Gamekeeper’s thumb, also known as “skier’s thumb,” is an injury of the ulnar collateral ligament of the thumb. Originally described as a chronic injury in Scottish gamekeepers, it is now commonly seen as an acutephase injury after sudden forced extension and abduction of the thumb against an object (e.g., ski pole) or as a football injury; this results in a partial or complete disruption of the ulnar collateral ligament of the thumb.1,2,3 Chronic injuries occur from repetitive thumb and hand movements, which result in gradually increasing laxity of the ulnar collateral ligament over time.1,2
Diagnosis
The diagnosis is suspected based on the clinical history and physical examination. Radiographs should be obtained to rule out underlying avulsion or condylar fractures.1,2 Once fracture has been ruled out, the thumb should be flexed at 30 degrees and stressed radially (abduction) to test for laxity and the lack of a defined end point. A complete tear should be considered if radial stress results in greater than 35 degrees of angulation, compared with the uninvolved side, with no defined end point.1,2,3
Clinical Complications
Management
For partial tears, a thumb spica splint should be applied for 2 to 6 weeks, after which flexion exercise is prescribed.1,2,3 Complete tears also should be placed in a thumb spica, although an early orthopedic referral is needed for possible surgical exploration and repair.1,2,3 The presence of an avulsion fracture larger than 5 mm, or a fracture involving the articular surface, also warrants early orthopedic follow-up for potential surgical treatment.1
REFERENCES
1. Richard JR. Gamekeeper’s thumb: ulnar collateral ligament injury. Am Fam Physician 1996;53:1775-1781.
2. Harrison BP, Hilliard MW. Emergency department evaluation and treatment of hand injuries. Emerg Med Clin North Am 1999;17:793-822.
3. Lee SJ, Montgomery K. Athletic hand injuries. Orthop Clin North Am 2002;33:547-554.
15-23 Bennett’s and Rolando’s Fractures
Colleen Campbell
Clinical Presentation
Patients present with pain at the base of the thumb after a direct axial compression injury, usually as a result of sports. Swelling and dorsal deformity of the metacarpal may be evident. Bony point tenderness at the base of the first metacarpal (MC) is present.
Pathophysiology
A Bennett’s fracture is an oblique, intraarticular fracture involving the volar edge of the first metacarpal base. A Rolando’s fracture is similar, except that the intraarticular component is “Y”- or “T”-shaped.1,2
Diagnosis
Diagnosis of Bennett’s or Rolando’s fractures usually is based on plain films of the finger, including anteroposterior and lateral views. The shaft of the MC appears dorsally dislocated, with the volar lip held in place. A posteroanterior stress view with the thumbs pressed
against each other at their radial borders reveals partial dislocations.2 Computed tomography may be necessary to fully visualize the articular surface.1
against each other at their radial borders reveals partial dislocations.2 Computed tomography may be necessary to fully visualize the articular surface.1
Clinical Complications
Complications include malunion, nonunion, chronic dislocation, arthritis, and chronic pain.
Management
Bennett’s fracture usually is treated by closed reduction, with Kirschner wiring used to fix the joint in abduction and extension.2 A cortical screw is used if the volar fragment is large (greater than 20% of the joint space).2 This is followed by immobilization in a thumb spica cast for 6 weeks.1 Treatment of Rolando’s fracture usually requires open reduction with multiple Kirschner wires through the trapezium and MC. Skeletal traction or external fixation occasionally is necessary for severely comminuted fractures.1 If the capsule is torn, it is reconstructed with the radial portion of the flexor carpi radialis tendon.2
REFERENCES
1. Palmer RE. Joint injuries of the hand in athletes. Clin Sports Med 1998;17:513-531.
2. Langford SA, Whitaker JH, Toby EB. Thumb injuries in the athlete. Clin Sports Med 1998;17:553-566.
15-24 Triquetral Fracture
Tyler Vadeboncoeur
Clinical Presentation
Pathophysiology
Dorsal cortical triquetral fractures occur by one of two mechanisms. A fall on the wrist in dorsiflexion and ulnar deviation can lead to impingement of the hamate or ulnar styloid on the dorsal triquetrum, or palmar flexion injury can cause the dorsal radiotriquetral and scaphotriquetral ligaments to produce a ligamentous avulsion of the dorsal cortex.3 Fractures of the triquetral body occur less frequently and are caused by a direct blow to the hand.2,3
Diagnosis
The diagnosis of a triquetral fracture depends on a high index of suspicion. A complete wrist series should be obtained. Dorsal chip fractures of the triquetrum often are seen on standard lateral radiographs of the wrist, but they are best seen on a pronated lateral view that projects the dorsal triquetrum away from adjacent carpal bones.3 Triquetral body fractures are best visualized on anteroposterior and oblique radiographs. There is localized tenderness over the dorsum of the wrist, just distal to the ulnar styloid.3
Clinical Complications
Management
REFERENCES
1. Bryan RS, Dobyns JH. Fractures of the carpal bones other than lunate and navicular. Clin Orthop 1980;149:107-111.
2. Hocker K, Menschik A. Chip fractures of the triquetrum: mechanism, classification, and results. J Hand Surg Br 1994;19: 584-588.
3. Cohen MS. Fractures of the carpal bones. Hand Clin 1997;13:587-599.
15-25 Carpal Tunnel Syndrome
Colleen Campbell
Clinical Presentation
Patients with carpal tunnel syndrome (CTS) present with wrist pain and a feeling that the hand is “falling asleep.” Symptoms may be worse at night and on awakening. Pain may radiate from the wrist to the proximal arm and forearm.1
Pathophysiology
CTS is an entrapment neuropathy of the medial nerve that causes pain, numbness, and weakness in the wrist and hand. The median nerve becomes entrapped by the overlying flexor retinaculum in the wrist. Repetitive motion, diabetes, connective tissue disorders, trauma, and pregnancy are associated with CTS. The digital sensory branch of the median nerve supplies sensation to the first three digits on the palmar aspect, and the thenar eminence is supplied by the palmar cutaneous sensory branch.2 The motor branch of the median nerve supplies the first and second lumbricals and gives off a recurrent thenar motor branch, which supplies the thenar muscles. The median nerve sensory branch supplies the lateral half of the ring finger as well.
Diagnosis
Diagnosis of CTS is based on history and physical examination findings. Classic findings in CTS include Tinel’s sign—increased pain and paresthesias when the flexor retinaculum is percussed over the median nerve at the wrist—and Phalen’s sign—exacerbation of symptoms when the patient actively flexes the wrists against each other for 30 to 240 seconds. Phalen’s sign is more sensitive and specific for CTS, but neither is highly sensitive nor specific.1,2 The diagnosis may be confirmed with evidence of prolonged sensory and motor latencies of the median nerve on electromyography (EMG). Up to 20% of patients have a “normal” EMG result unless internal comparison myography is performed on the ulnar or radial nerve.2 Extremes of wrist flexion or extension may exacerbate the pain. The thenar eminence may be visibly atrophied, with weakness noted in thumb abduction and opposition.2
Clinical Complications
Complications include chronic weakness of the thenar muscles and associated disability.
Management
Treatment involves limiting repetitive activities. Wrist splints that hold the wrists in neutral position and a short course of nonsteroidal antiinflammatory drugs (NSAIDs) may be helpful. Depo-Medrol injections adjacent to the carpal tunnel may be used only once or twice to alleviate symptoms. Surgical decompression of the carpal tunnel is successful in 90% of refractory cases.2
REFERENCES
1. Campbell WW. Diagnosis and management of common compression and entrapment neuropathies. Neurol Clin 1997;15:549-567.
2. Preston DC. Distal median neuropathies. Neurol Clin 1999;17:407-424.
15-26 DeQuervain’s Tenosynovitis
Colleen Campbell
Clinical Presentation
Patients with DeQuervain’s tenosynovitis present with pain in the base of the thumb that is exacerbated by repetitive motion. There is pain on palpation of the first dorsal compartment of the hand. In some cases, crepitus or triggering may be evident with thumb movement. If symptoms are chronic, the tendons may be noticeably thickened, and there may be a ganglion cyst.1
Pathophysiology
DeQuervain’s disease is a stenosing tenosynovitis that involves the abductor pollicis longus (APL) and the extensor pollicis brevis (EPB) of the thumb. DeQuervain’s is the most common tendonitis of the wrist in athletes.1 Repetitive activities that involve forceful grasping with ulnar deviation cause this type of tendonitis. Initially, the condition was described in middle-aged washer women.2 People involved in activities such as golf, fly fishing, and racquet sports (squash, racquetball) may be susceptible.2 It is unclear which is inflamed first, the tendon and synovium or the overlying sheath.1
Diagnosis
The diagnosis is made on the basis of the history and physical examination. There are two tests that help identify this disease process. In Finkelstein’s test, which is considered pathognomonic, maximal pain is elicited when the thumb is held flexed in the fist while the wrist is pulled into ulnar deviation by the examiner.1 In the alternative test, the wrist is held in radial deviation while the examiner actively resists thumb extension.
Clinical Complications
Complications from DeQuervain’s tenosynovitis include weakened grip strength and disability from pain.
Management
Initial treatment of DeQuervain’s tenosynovitis involves application of ice, rest, and use of nonsteroidal antiinflammatory drugs (NSAIDs). A thumb spica splint is indicated for persistent symptoms. One or two injections of corticosteroids (0.5 mL of dexamethasone and 0.5 mL of lidocaine 2%) in the first dorsal compartment, administered 6 weeks apart, usually provide relief of symptoms. Rehabilitation measures include stretching and strengthening of the APL and EPB tendons. Surgical release of the sheath is reserved for those rare cases in which conservative measures fail.1
REFERENCES
1. Rettig AC. Wrist and hand overuse syndromes. Clin Sports Med 2001;20:591-611.
2. Fulcher SM, Kiefhaber TR, Stern PJ. Upper-extremity tendinitis and overuse syndromes in the athlete. Clin Sports Med 1998;17:433-448.
15-27 Colles’ and Smith’s Fractures
Mark Silverberg
Clinical Presentation
Most patients with Colles’ or Smith’s fracture patterns present after low-energy mechanisms such as a fall onto an outstretched hand. Patients complain of pain in the distal forearm and may or may not have an obvious deformity. If a Colles’ fracture is grossly displaced, some refer to it as a “dinner fork” deformity, because its bent appearance resembles the shape of a fork.1
Pathophysiology
Colles’ fracture is an extraarticular fracture of the distal forearm with dorsal displacement of the distal fragment, whereas Smith’s fracture is an intraarticular or extraarticular fracture of the same region with palmar displacement of the distal wrist.1,2 The mechanism of injury is similar in both types; both are usually caused by a fall onto an outstretched hand. Many patients presenting with these injuries are elderly women with some degree of osteoporosis. Osteoporosis is thought to contribute substantially to the fragility of the distal radius, making the elderly female population more likely to sustain these sorts of fractures.1
Diagnosis
Clinical Complications
Soft tissue injuries of the cartilages, ligaments, and joint capsule may not be seen on radiographs but can make realignment difficult.2 Injuries to tendons or nerves (most commonly the median nerve) may be present and may necessitate open reduction with internal fixation and simultaneous carpal tunnel release.1 Vascular compromise rarely occurs, but nonunion, malunion, and post-traumatic arthritis are common complications.1,2
Management
In the event of an open fracture, immediate surgical treatment is necessary.2 If doubt exists regarding the complexity of the fracture and the treating physician is unsure whether surgical treatment is necessary, a computed tomographic scan may be helpful.1 Treatment modalities include closed reduction with plaster immobilization and open reduction with internal fixation.1,2 After closed reduction, radiographs should be repeated to ensure acceptable fragment alignment.1,2 Neurologic status should be checked carefully before and after reduction, because median nerve injuries are common with these fractures.1,2
REFERENCES
1. Hanel DP, Jones MD, Trumble TE. Wrist fractures. Orthop Clin North Am 2002;33:35-57.
2. Hanker GJ. Radius fractures in the athlete. Clin Sports Med 2001;20:189-201.
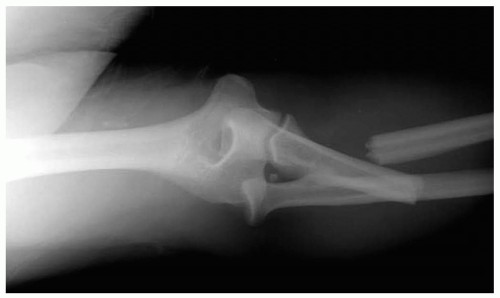
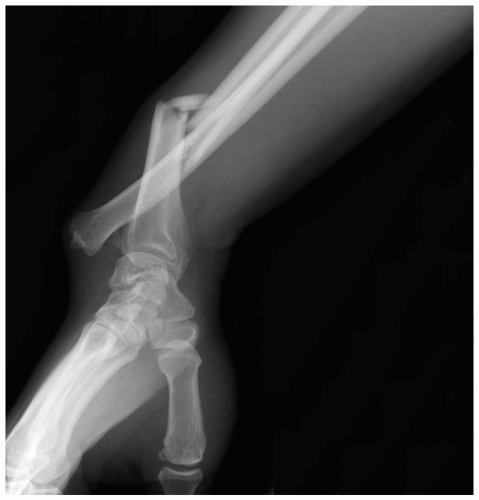
15-28 Monteggia Fracture-Dislocation
Anthony S. Mazzeo
Clinical Presentation
Patients with Monteggia fracture-dislocations (MFDs) present with forearm pain after a fall or direct trauma.
Pathophysiology
The MFD injury involves a proximal ulnar fracture associated with dislocation of the radial head. Because of the close proximity of the radius and ulna, forces that cause fracture of one bone may result in dislocation of the proximate neighboring bone.1,2 In MFD, the annular ligament and the interosseus membrane are often injured as well. In cases of falls, the mechanism is most often a fall onto an outstretched hand with the hand pronated or the elbow flexed. Trauma to the proximal forearm, such as from a “nightstick injury” (in which the recipient attempts to block a downward-moving blow from a hard, stick-like object), may also result in MFD.1,2
Diagnosis
Radiographic imaging reveals the proximal ulnar fracture and should then prompt an assessment of the radial head. A true lateral radiograph of the elbow may demonstrate the radial head dislocation. A line drawn through the center of the radial head should pass through the center of the humeral capitellum.1 Comparison views may be necessary in some cases. In addition to diagnosing MFD, the physician must perform a complete neurovascular examination to rule out associated limb-threatening injury and to document nerve function.1,2
Clinical Complications
Management
The initial reduction and treatment regimen must be determined in conjunction with an orthopedic surgeon, because of the potential for long-term pain and disability if this injury is not appropriately managed.1 Most MFDs in adults require open reduction and operative repair. Most pediatric cases heal well with a closed approach after appropriate reduction.2 Adequate analgesia is essential, and antibiotics should be administered if there is an open fracture.1,2
REFERENCES
1. Perron AD, Hersh RE, Brady WJ, Keats TE. Orthopaedic pitfalls in the ED: Galeazzi and Monteggia fracture-dislocation. Am J Emerg Med 2001;19:225-228.
2. Wilkins KE. Changes in the management of Monteggia fractures. J Pediatr Orthop 2002;22:548-554.
15-29 Galeazzi Fracture-Dislocation
Anthony S. Mazzeo
Clinical Presentation
Pathophysiology
GFDs involve fractures of the radius at the junction of the middle and distal thirds, with concomitant dislocation.1 In most cases, the dislocation is dorsal. Because of the anatomic proximity of the radius and ulna, forces that are capable of fracturing one bone may result in dislocation of the proximate neighboring bone.
Diagnosis
Physical examination reveals pain and swelling at the radial fracture site. Visible deformity or swelling over the DRUJ may also be present.1,2 Radiographs reveal the fracture near the junction of the distal and middle thirds of the radius. The presence of this fracture should prompt the clinician to look closely for DRUJ dislocation. Radiographic evidence of DRUJ dislocation, however, may be subtle.1,2 DRUJ dislocation should be suspected if radiographs reveal either shortening of the radius compared with the ulna, or a space greater than 2 mm between the two bones on anteroposterior view.1 Comparison views are often helpful. Computed tomographic imaging may help in assessing for DRUJ dislocation.
Clinical Complications
A complete vascular and neurosensory examination must be done in all cases. The anterior interosseus branch of the median nerve, which innervates the flexor pollicis longus and flexor pollicis brevis, is susceptible to injury and should be assessed carefully.1,2 Physical examination reveals a weak pinch between the index finger and the thumb if this nerve is injured. Without prompt surgical intervention, GFD has a high rate of complications, including malunion, nonunion, nerve injuries, limitations in pronation and supination, chronic pain, and weakness.1 Compartment syndrome is a rare complication that must be watched for.1,2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree