Opioids
Jeffrey Uppington
Among the remedies which it has pleased Almighty God to give to man to relieve his sufferings, none is so universal and so efficacious as opium.
—Thomas Sydenham, 1624–1689
Opioids are the core pharmacologic treatment of pain. They are the mainstay for treatment of both acute pain and cancer pain, and although controversy still exists over their use in chronic nonterminal pain (CNTP), they are increasingly used for this indication also. Opioids are the only pain medications that have no ceiling effect and are therefore the only systemic treatment that can be used to treat severe accelerating pain. Any health care provider treating pain should understand the effects and proper usage of these important drugs.
I. TERMINOLOGY
Opiates are drugs derived from opium, which is obtained from the juice of the poppy Papaver somniferum. These drugs include morphine, codeine, and various semisynthetic congeners derived from morphine and codeine, and other components of opium such as thebaine. The term opioid applies to substances with morphine-like activity, including agonists and antagonists, as well as naturally occurring and synthetic opioid peptides. Endorphin is a generic term applying to the endogenous opioid peptides. There are three families of endogenous opioids—the endorphins, the enkephalins, and the dynorphins. The word narcotic was derived from the Greek word for stupor. Originally, narcotic referred to any drug that induced sleep, but it later became associated with the strong opiate analgesics. The term is no longer useful pharmacologically because it is being increasingly used in the legal and regulatory context to refer to a wide variety of abused substances.
II. ENDOGENOUS OPIOIDS
Each of the three families of opioid neuropeptides (i.e., endorphins, enkephalins, and dynorphins) is derived from a distinct precursor polypeptide and has a distinct anatomic distribution. Like peptide hormones, endogenous opioids have biologically inactive precursors that generate active agents only after enzymatic cleavage. The precursor for β-endorphin, proopiomelanocortin, also contains peptide sequences for adrenocorticotropin (ACTH) and melanocyte-stimulating hormone (MSH), illustrating the close relation between the endogenous opioids and hormone systems.
III. CLASSIFICATION OF OPIOIDS
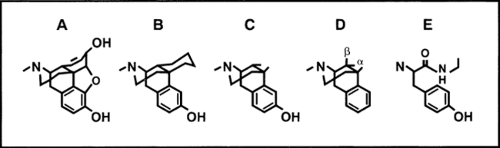
Opioids can be classified as naturally occurring, semisynthetic, and synthetic (see Table 1). Morphine, codeine, papaverine, and thebaine are naturally occurring. The semisynthetic drugs are derived from morphine, codeine, and thebaine. The synthetic drugs structurally resemble morphine but do not occur in nature. They are produced by gradually reducing the number of
rings from the five-ring structure of morphine, through the four-ring “morphinans,” the three-ring “benzomorphinans” to the two-ring “phenylpiperidines” (see Fig. 1). There are alternative classifications of opioids. The drugs may be grouped according to the specific receptors they act on (see subsequent text). Another useful distinction is whether they are agonists, antagonists, or some combination of the two (see Table 2).
rings from the five-ring structure of morphine, through the four-ring “morphinans,” the three-ring “benzomorphinans” to the two-ring “phenylpiperidines” (see Fig. 1). There are alternative classifications of opioids. The drugs may be grouped according to the specific receptors they act on (see subsequent text). Another useful distinction is whether they are agonists, antagonists, or some combination of the two (see Table 2).
Table 1. Classification of opioids | |
|---|---|
|
IV. OPIOID RECEPTORS
Opioids act via specific receptors on cell membranes. Specific opioid receptors have been proposed through a mixture of clinical and laboratory observation. The structure of opioid receptors is currently understood at cellular, molecular, and genetic levels. It is now clear that there are three well-defined “classical” opioid receptors (i.e., μ, δ, and κ). Recently, DNA encoding for an “orphan” receptor has been identified. The “orphan” receptor has a high degree of similarity to the “classical” opioid receptors and has been named opioid receptorlike (ORL). There is also pharmacologic evidence for subtypes of each known receptor, and for
other, less well-characterized opioid receptors, including α and γ. The ν receptor is no longer considered to be an opioid receptor.
other, less well-characterized opioid receptors, including α and γ. The ν receptor is no longer considered to be an opioid receptor.
Table 2. Alternative classification of opioids | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
1. μ Receptors
It seems likely that morphine and morphinelike drugs produce analgesia primarily through interaction with μ-receptors. These receptors are present in large quantities in the periaqueductal gray matter (brain) and the substantia gelatinosa (spinal cord). Activation of μ receptors results in analgesia, euphoria, respiratory depression, nausea and vomiting, decreased gastrointestinal (GI) motility, tolerance, and dependence. β-endorphin has a high affinity for μ receptors, as do the enkephalins. Dynorphin also binds to the μ-receptor but not as avidly as it does to the κ receptor.
The proposed classification into μ1 and μ2, which provided a rationale for the hope that selective μ1 agonists could provide analgesia without respiratory depression, did not survive the scrutiny of several laboratories.
2. κ Receptors
Activation of these receptors also causes analgesia, but less respiratory depression than μ-receptor activation. κ receptor activation produces dysphoria and hallucinations rather than euphoria. Several κ receptor subtypes have been proposed as a result of binding studies, but their actions have not been fully elucidated. Dynorphin A is the endogenous ligand for the κ receptor.
3. δ Receptors
Using selective agonists and antagonists, studies have established δ-receptor analgesia both spinally and supraspinally, although the spinal system analgesia appears more robust. δ1– and δ2-receptors have been proposed, based on differential sensitivity to several antagonists. The enkephalins are the endogenous ligands for the δ-receptors.
4. Opioid Receptor–like Receptors
Although the ORL receptor is accepted as a member of the opioid receptor “family” because of its structural similarity to the “classical” opioid receptors, there is no pharmacologic similarity. The receptor was called an “orphan” receptor because ligands of the “classical” receptors did not have the same high affinity for the “orphan” receptors. A confusion of effects of known high-affinity ligands has been reported, including antinociception, pronociception/hyperalgesia, allodynia, and no effect.
5. Cloned Receptors
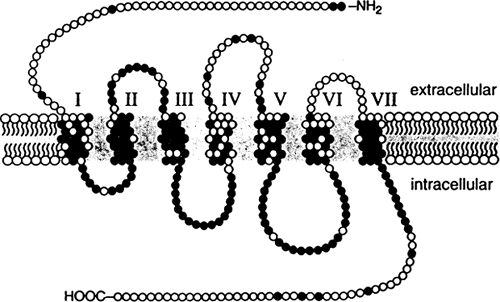
The μ, δ, κ, and ORL receptors are the only opioid receptors whose genes have been identified and have been cloned, despite an intensive search for genes corresponding to opioid receptor subtypes and the less well-characterized receptors. The cloned opioid receptors have characteristics of typical G-protein–coupled receptors. There are seven hydrophobic regions that span the cell membrane, with three extracellular and three intracellular loops. There is an intracellular carboxy-terminal tail and an extracellular amino-terminal tail. The amino acid sequences of the different opioid receptors are approximately 65% identical to each other. The regions of highest similarity are the sequences predicted to lie in the seven transmembrane spanning regions and intracellular loops. The extracellular regions that differ in amino acid sequence may contain the unique ligand-binding domains for each receptor (see Fig. 2).
6. Receptor Mechanisms
Opioid receptors are coupled to G-proteins and are therefore able to affect protein phosphorylation via second messenger systems, thereby altering ion channel conductance. Opioids act both presynaptically and postsynaptically. Presynaptically, they inhibit the release of neurotransmitters, including substance P and glutamate. Postsynaptically, they can inhibit neurons by opening potassium channels that hyperpolarize the cell. There is evidence that opioids produce both short- and long-term effects on neural function. Opioids may play a distinct role during early embryonic development. Administration of opioids considerably reduces the facilitation of nociceptive processing (e.g., “windup”). Opioids (including endogenous opioids) can also affect opioid gene regulation with possible short-term and long-term effects, and local as well as distal effects.
7. Alternative Opioid Mechanisms
Not all nociceptive mechanisms are mediated by opiate receptors. It is known that N-methyl-D-aspartate (NMDA)-sensitive glutamate receptors are involved in nociceptive transmission in the spinal dorsal horn. Norepinephrine, serotonin, and sodium channels are also involved, and it is possible that a central nitric oxide–cyclic guanosine monophosphate signaling pathway may help mediate nociception. It appears that some opioid actions are not mediated by opioid receptors, and this is a potentially important observation in terms of understanding pain and analgesic mechanisms. Therefore, methadone, meperidine, and tramadol inhibit serotonin and norepinephrine reuptake. Methadone, meperidine, and other opioids are antagonists of the NMDA amino acid excitatory pathway. Meperidine blocks sodium channels and has local anesthetic properties.
V. OPIOID EFFECTS
1. Central Nervous System
(i) Analgesia, Mood, and Consciousness
Opioids selectively relieve pain without affecting other sensory modalities. Pain can be described as a specific sensation (i.e., burning, shooting, throbbing) or in terms of suffering (i.e., excruciating and miserable). Opioids alter the sensation of pain as well as the affective response. Patients often say that their pain is still present but that they feel more comfortable. Occasionally, patients experience euphoria or dysphoria, more so when these drugs are used for
recreational purposes. Useful analgesia occurs without loss of consciousness, although high doses of opioids do produce unconsciousness, and drowsiness is a common side effect.
recreational purposes. Useful analgesia occurs without loss of consciousness, although high doses of opioids do produce unconsciousness, and drowsiness is a common side effect.
(ii) Respiratory Depression
Opioids of the morphine type depress respiration by acting directly on the respiratory centers in the brain stem. Equianalgesic doses of morphinelike opioids produce the same degree of respiratory depression as morphine itself. Partial agonists and agonist-antagonist opioids are less likely to cause severe respiratory depression, as are the selective κ-agonists. Therapeutic doses of morphine depress all phases of respiration, respiratory rate, and minute volume. At the same time, the responsiveness to carbon dioxide (CO2) is decreased and, therefore, the CO2 response curve is shifted upward and to the right. The degree of respiratory depression is dependent on opioid dose and patient status, and apnea is a true risk. Pain and stimulation counteract respiratory depression, while sedative drugs, such as the benzodiazopines, potentiate it. Natural sleep also reduces CO2 responsiveness and is additive to the opioid effect. Unexpected respiratory depression may occur in relation to variations in serum concentration, concomitant drug use, and varying degrees of pain and stimulation. Naloxone effectively reverses the respiratory depression.
(iii) Nausea and Vomiting
Nausea and vomiting from opioids is due to the direct stimulation of the chemoreceptor trigger zone (CRTZ). The CRTZ is situated in the area postrema in the floor of the 4th medulla. There is also an associated increase in vestibular sensitivity so that opioid-induced nausea tends to be exacerbated by movement. Treatment includes opioid dosage reduction, antidopaminergics (e.g., droperidol, compazine, metoclopramide), anticholinergics (e.g., scopolamine), or serotonin antagonists (e.g., ondansetron).
(iv) Cough
Opioids depress the cough center in the medulla. There is no relation between cough suppression and respiratory depression, and effective antitussive agents are available that do not depress respiration in clinical doses, such as dextromethorphan. The antitussive receptors are less sensitive to naloxone than receptors involved in analgesia.
(v) Miosis
μ and κ-agonists constrict the pupil by excitating the Edinger-Westphal nucleus (parasympathetic) of the oculomotor nerve. Tolerance to the miotic effects occurs with long-term opioid use, but addicts, with high circulating blood concentrations of opioids, will have small pupils. The pupillary effects of opioids are altered by concomitant use of drugs, including general anesthetics. Morphine reduces intraocular pressure.
(vi) Convulsions
In animals, high doses of morphine and related opioids cause convulsions. The drugs stimulate hippocampal pyramidal cells, probably by inhibiting release of γ-aminobutyric acid (GABA) at the synaptic level. Selective δ-agonists may do the same. In
humans, convulsions are rarely seen because seizure-producing doses are extremely high and not given. However, meperidine is particularly prone to produce seizure activity through its metabolite normeperidine, the accumulation of which is most likely to occur in patients with renal dysfunction and in the elderly. Meperidine-induced seizures are relatively common, and for that reason the use of meperidine is discouraged, particularly in susceptible patients, and for chronic pain. Naloxone can be used to treat seizures but is more effective in treating convulsions caused by morphine and related drugs than meperidine.
humans, convulsions are rarely seen because seizure-producing doses are extremely high and not given. However, meperidine is particularly prone to produce seizure activity through its metabolite normeperidine, the accumulation of which is most likely to occur in patients with renal dysfunction and in the elderly. Meperidine-induced seizures are relatively common, and for that reason the use of meperidine is discouraged, particularly in susceptible patients, and for chronic pain. Naloxone can be used to treat seizures but is more effective in treating convulsions caused by morphine and related drugs than meperidine.
(vii) Hypothalamic Effects
Opioids can cause decrease in body temperature. The chief mechanism is alteration of the equilibrium point of the hypothalamic heat-regulating mechanism, although opioid-induced vasodilation may worsen the effect. Although shivering is not observed consistently after opioid anesthesia, it does occur frequently after inhalation anesthesia. Small doses of opioid (particularly meperidine) can attenuate or abolish this shivering through a mechanism that is poorly understood.
2. Neuroendocrine Effects
Opioids have a number of neuroendocrine effects. High-dose opioid therapy reduces release of stress hormone (i.e., glucocorticoids and catecholamines). It is not at all clear to what extent and under which conditions this is a desirable effect. Evidence is emerging that high-dose opioids may also suppress immune response, which clearly is not a desirable effect. Opioids suppress hypothalamic releasing factors, thereby suppressing the release of lutenizing hormone (LH), follicle-stimulating hormone (FSH), ACTH and β-endorphin. Cortisol and testosterone levels are thereby reduced. In women, the menstrual cycle may be disrupted, and testosterone levels may be reduced in men. Some opioids also reduce growth hormone production. During chronic opioid administration, tolerance to these effects develops. Therefore, when heroin addicts are maintained on methadone, the disrupted menstrual cycles and plasma concentrations of LH of women and the depressed testosterone levels of men return to normal.
3. Gastrointestinal System
(i) Stomach
Gastric motility is decreased, prolonging gastric emptying and increasing the risk of esophageal reflux. The passage of gastric contents through the duodenum is usually delayed. μ agonists usually decrease gastric acid secretion, but stimulation can occur. Indirect effects, such as increased secretion of pancreatic somatostatin, predominate.
(ii) Small Intestine
Biliary, pancreatic, and intestinal secretion are diminished, and the digestion of food is delayed. The duodenum is affected more than the ileum; water is absorbed more completely and the viscosity of bowel contents increases.
(iii) Large Intestine
Peristaltic propulsive waves are decreased or abolished in the colon. Bowel tone increases. Water is absorbed, which desiccates the
feces and slows their passage. In postoperative patients, prolonged ileus is a problem; in patients on long-term opioids, constipation is common and these patients should take stimulant laxatives.
feces and slows their passage. In postoperative patients, prolonged ileus is a problem; in patients on long-term opioids, constipation is common and these patients should take stimulant laxatives.
(iv) Biliary Tract
The sphincter of Oddi constricts, and bile duct pressure may increase. Despite this, little clinical effect is seen. Naloxone reverses the effects, as does glucagon. Atropine and nitroglycerine only partially reverse the effect. Morphine and morphine-like drugs are thought by some to have a less pronounced effect than meperidine and its derivatives, but the validity of this finding is in doubt.
4. Cardiovascular System
Opioids have a number of actions on the cardiovascular system. Histamine release and peripheral vasodilation accompany the use of morphine and of some other opioids. High doses of any opioid will reduce sympathetic output, and thus allow a greater preponderance of parasympathetic effects. The pulse rate may be slowed by stimulation of the vagal center, especially with high doses. There is little direct effect on the myocardium, but the peripheral effects may reduce myocardial oxygen consumption, left ventricular end diastolic pressure, and cardiac work. High doses, low blood volume, and the combination of other drugs such as phenothiazines accentuate the hypotensive effects.
5. Tolerance, Dependence, and Addiction
These phenomena are discussed in detail in Chapters 30 and 35. However, it is important to understand these phenomena when prescribing opioids, so a brief overview is included here. Although tolerance and physical dependence are likely, or almost inevitable, consequence of chronic opioid use, addiction is a behavioral problem that arises only in certain individuals. Tolerance is marked by the need for increasing doses to achieve the same analgesic effect and is a form of tachyphylaxis. Tolerance to side effects, other than bowel effects, also occurs. Changing from one opioid to another is often effective in reducing tolerance because of incomplete cross-tolerance between opioids (see Chapter 30). Physical dependence




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree