II.Asthma
A. Introduction
1. Asthma presentation and symptoms. Asthma causes episodic partially reversible airway obstruction resulting from bronchoconstriction, inflammation, hyperresponsiveness, and mucous plugging. Symptoms include wheezing, chest tightness, difficulty breathing, and coughing. These symptoms may be exacerbated by exercise, viral infections, weather, pain, stress, and exposure to various allergens.
2. Epidemiology. Asthma affects up to 12% of pregnant women worldwide and the prevalence is rising. It is one of the most common medical conditions complicating pregnancy and the most common respiratory disease affecting parturients.1
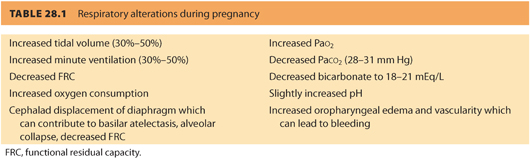
B. Effect of pregnancy on asthma. The asthmatic patient may be adversely affected by the physiologic changes in pregnancy. These include decreased functional residual capacity (FRC), increased minute ventilation, increased oxygen consumption, and elevation of the diaphragm by the gravid uterus.
1. Exacerbations. Approximately 20% to 40% of parturients with asthma experience exacerbations or increased severity, which require medical intervention.1,2 Women with severe asthma tend to have a higher exacerbation rate.3
a. Timing of exacerbations. Exacerbations usually occur in the late second trimester.1
b. Risk factors. Risk factors for exacerbation most commonly include viral infections and noncompliance with medication use.1,4,5
2. Improved or unchanged symptoms. Approximately 23% to 30% of patients have an improvement in asthma severity. Maselli et al.2 reported that 30% of patients had no change in their symptoms.
3. Symptoms during labor and delivery. Acute attacks during labor and delivery are not common.1 If they do occur, they tend to be mild. Women with severe asthma are more likely to suffer symptoms during labor than women with mild asthma.
C. Effect of asthma on pregnancy
1. Asthma and maternal pregnancy complications. Asthma has been associated with a number of adverse pregnancy complications.
a. A large recent review and meta-analysis estimated a 31% increased risk for cesarean delivery (CD) and a 40% increased risk for gestational diabetes.6 Another study found that the incidence of preeclampsia was increased by 50% in women with asthma.7 Not all authors agree on particular maternal complications, but those frequently cited include preeclampsia, gestational diabetes, hemorrhage, placental abruption, premature rupture of membranes (PROM), increased CD rates, placenta previa, preterm labor (PTL), and preterm delivery (PTD).3,4,6,7
b. Maternal complications and asthma severity. Some maternal and placental complications are attributed to asthma severity.6–8 In a large recent review and meta-analysis, moderate to severe asthma was associated with an increased pregnancy risk, whereas optimal management decreased the risk.4,6
2. Fetal and neonatal complications. Neonatal complications associated with asthma include increased perinatal morbidity and mortality, PTL, PTD, low birth weight (LBW), small for gestational age (SGA) infants, and possible increase in fetal congenital malformations.4,7
a. Congenital malformations. There are some reports of congenital malformations of the cardiac, respiratory, nervous, digestive, and dermatologic systems in the offspring of parturients with asthma.9 Murphy et al.,5 in a large review and meta-analysis, reported that women with asthma are 11% more likely to have fetal congenital malformations. These malformations were not associated with exacerbations or the use of inhaled bronchodilators. Further studies will be needed to confirm these findings and to understand the mechanism.
b. Neonatal complications and asthma severity. Some neonatal complications correlate with asthma severity and exacerbations.1,4,7,8,10 For example, in a large review and meta-analysis, there was an increased risk for LBW and SGA infants in moderate to severe, compared to parturients with mild asthma. Women with asthma with acute exacerbations were at greater risk for PTD and LBW infants. Many neonatal risks can be reduced to nonsignificant levels with active asthma management.7
D. Asthma classification and control
1. Pulmonary function tests. Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), the FEV1/ FVC ratio, peak expiratory flow rate (PEFR), and the mean forced expiratory flow (FEF) during the middle half of FVC (FEF25–75) are all useful measurements in the management of pregnant women with asthma. Typically, asthma control is assessed by FEV1 and PEFR. More recently, the fraction of exhaled nitric oxide has been used successfully to guide management.2
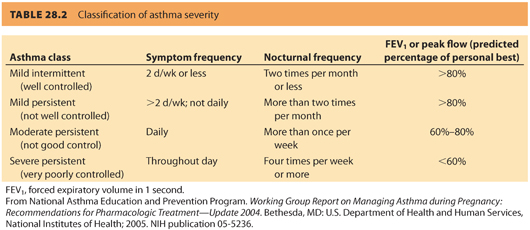
2. Asthma severity classification. The definition of asthma class (severity) and control according to the National Asthma Education and Prevention Program (NAEPP) is summarized in Table 28.2. Asthma severity is classified by symptom frequency and measurement of FEV1 and PEFR for patients on or off medications for asthma control.11
3. Remove triggers. As mentioned earlier, exacerbations tend to occur in patients who discontinue medications and in those with severe disease.3,8 It is important to remove environmental triggers, treat infections, control allergic rhinitis and gastroesophageal reflux (GERD), and institute medical management.1
4. Obstetric testing. In addition to good control of asthma symptoms with medications, obstetric management will require serial ultrasonography and other antenatal testing. Pulmonary function tests (PFTs) and PEFR should be followed regularly.
E. Medical management. Asthma control is necessary to improve both maternal and fetal outcomes.2–4
1. Medication use in pregnancy. Medications should be administered when necessary and are considered to be safe during pregnancy.2,3,10 There is some controversy over oral corticosteroid use during the first trimester and an increased incidence of LBW and cleft lip, with or without cleft palate.3 Corticosteroids may be lifesaving for the severe asthmatic and should be given when needed.
CLINICAL PEARLIn general, the benefits of asthma medication use in pregnancy outweigh the risk of exacerbations and other complications from asthma or from the use of asthma medication itself.
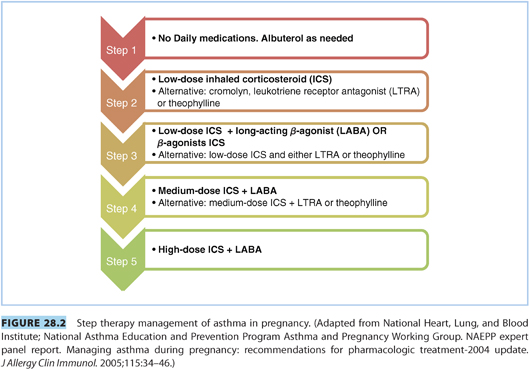
2. Step approach to treatment. The NAEPP recommends that patients be treated in a step-up fashion, adding medications depending on asthma severity. The most commonly prescribed medications are anti-inflammatory inhaled corticosteroids and inhaled β2-agonists. See Figure 28.2 for the step approach to treatment recommended by the NAEPP.
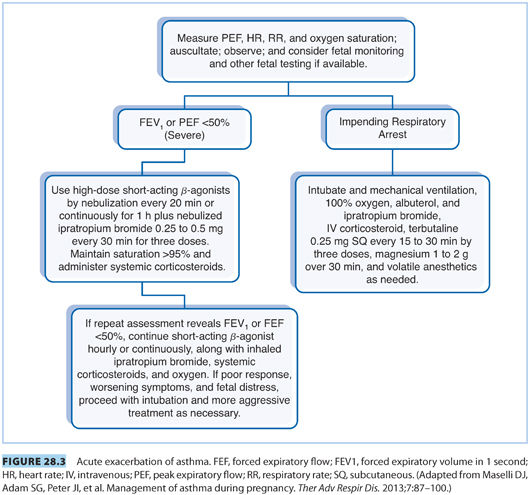
F. Labor management. See Figure 28.3 for the management of an acute exacerbation of asthma during labor or delivery.2
1. Induction of labor. Cervical ripening with prostaglandins for the induction of labor should be undertaken with caution. Prostaglandin F2α is a bronchoconstrictor and its use during labor has been associated with bronchospasm. Prostaglandin E2 has been associated with bronchodilation and bronchoconstriction.12 β-Adrenergic receptor blockade can cause bronchospasm and should be used with caution.13
2. Anesthetic goals. Anesthetic goals include good analgesia, prevention of stress and increased minute ventilation, and avoidance of a high sensory or motor block. The latter might interfere with the muscles of respiration and could also lead to unopposed parasympathetic-induced bronchoconstriction.13
3. Analgesia for labor
a. Opioids. Opioids should be administered carefully to patients with active asthma, given the potential for respiratory depression and bronchoconstriction. Histamine-releasing opioids such as morphine and meperidine should be used with caution. Fentanyl and remifentanil may be better choices.13
b. Neuraxial analgesia. Pudendal and paracervical blocks can be helpful, but neuraxial analgesia has many advantages. Hyperventilation, pain, and stress can aggravate asthma during labor.
(1) Benefits. Neuraxial analgesia benefits the asthmatic parturient by decreasing oxygen consumption, work of breathing, stress, and minute ventilation during labor. In addition, an epidural can be extended for CD in an emergency so that manipulation of the airway, and resultant bronchospasm, can be avoided.13,14
(2) Pulmonary effects. A dilute solution of epidural local anesthetic and opioid to a T10 level for labor should have minimal motor block and a small effect on respiratory function.15 Lumbar epidural analgesia for labor is unlikely to adversely affect respiration. There are reports of only a 3% decrease in vital capacity (VC) with a motor block to approximately T8, which is a higher level and denser blockade than would be required for labor.14
G. Anesthesia for cesarean delivery
1. Neuraxial anesthesia. Neuraxial anesthesia is preferable to general endotracheal anesthesia (GETA) given the risk of provoking bronchospasm with GETA. This is especially important during general anesthesia where a rapid sequence induction (RSI) is required and there is a tendency to give less volatile anesthetic after delivery, which could result in lighter levels of anesthesia and precipitate bronchospasm.15
a. Anesthetic level and asthma exacerbation. There is concern that high levels of blockade may impair accessory respiratory muscle function in women with severe asthma and that a sympathectomy could lead to unopposed parasympathetic tone and bronchospasm.14,15 A carefully titrated epidural or a low-dose combined spinal-epidural may be preferable to a spinal anesthetic in patients with severe asthma.15 Although there are concerns about the adverse pulmonary effects from a high thoracic blockade, such a block appears safe and to have minimal effects on pulmonary function in patients with chronic obstructive pulmonary disease (COPD).
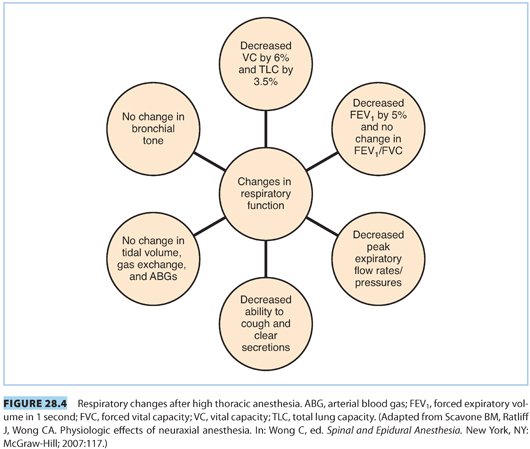
b. Effects of thoracic neuraxial anesthesia. In normal parturients, high thoracic epidural anesthesia reduces VC by 6%, total lung capacity (TLC) by 3.5%, and FEV1 by 5%. The ratio of FEV1 /FVC does not change. There is no change in tidal volume (TV), gas exchange, arterial blood gases (ABGs), the ventilatory response to hypoxemia or hypercarbia, or bronchial tone. There does appear to be a decrease in PEFR after spinal anesthesia in parturients and a decrease in peak expiratory pressure (PEP) after epidural anesthesia, although this depends on the density of the thoracic motor block and does not seem to affect patients with COPD.16 Respiratory impairment from high thoracic neuraxial anesthesia appears to be minimal in patients with COPD.14,16 See Figure 28.4 for a summary of these findings.
c. Postoperative management. Patients having neuraxial anesthesia can enjoy improved postoperative pain management via epidural analgesia or with long-acting neuraxial opioids, such as preservative-free morphine. This could lead to less postoperative pulmonary dysfunction than after GETA.13,14
2. General endotracheal anesthesia. Should GETA become necessary, inhaled β2-agonists and intravenous (IV) lidocaine may reduce airway hyperactivity, but the former may decrease uterine tone.13,14
a. Induction agents. Ketamine is the preferred induction agent because of its sympathomimetic bronchodilating properties; however, it does increase secretions. Propofol decreases the response to airway manipulation and may have direct airway smooth muscle relaxant activity, making it a good alternative.15
b. Muscle relaxants. Neuromuscular blocking agents that release histamine, such as curare and atracurium, should not be used. Most of the muscle relaxants in use today will not cause bronchospasm; however, neuromuscular blocking agents are common medications implicated in allergic reactions in the operating room.13
c. Reversal agents. Reversal agents such as neostigmine may cause bronchospasm and increase airway secretions. They should be used carefully and after administration of an anticholinergic agent.13,15
d. Aspiration risk. Although inhalational induction by mask ventilation and deep extubation are sometimes performed in women with severe asthma, the risk of pulmonary aspiration is increased in parturients. Thus, parturients with asthma should typically be extubated awake. The risk of bronchospasm must be weighed against the risk of aspiration.
3. Postpartum hemorrhage. Postpartum hemorrhage (PPH) is best treated with oxytocin. Ergot alkaloids have been linked to bronchospasm, and prostaglandin F2α is relatively contraindicated because it can precipitate bronchospasm. Prostaglandin E2 aerosols can provoke bronchospasm as well. Prostaglandin E1 appears to be safer. Other means of controlling hemorrhage, such as embolization and arterial ligation, should be considered.10,15
CLINICAL PEARLDrugs that can precipitate bronchospasm include opioids, β-blockers, histamine-releasing neuromuscular blockers, reversal agents, ergot alkaloids, and prostaglandin F2α.
III.Pulmonary embolism during pregnancy
A. Introduction. Embolic diseases during pregnancy include pulmonary thromboembolism (PE), AFE, and venous air embolism (VAE). Approximately one-fifth of deaths in the United States are due to embolic disease and constitute obstetric emergencies.17 PE is one of the most common causes of maternal mortality worldwide. Venous thromboembolism (VTE) manifests as deep vein thrombosis (DVT) and PE. DVT is more common (75% to 80% of VTE) than PE (20% to 25%).18,19
1. Venous thromboembolism prevalence and mortality. VTE occurs in approximately 0.6% to 1.8% per 1,000 pregnancies and accounts for 1.1 to 1.6 deaths per 100,000 births.20,21 Recent studies report that 10.2% of pregnancy-related deaths in the United States are due to VTE; however, some report a mortality rate as high as 20% to 25% in the United States.22 PE alone occurs in 0.4 to 0.5 per 1,000 births,23 and 80% of women with PE will have evidence of a DVT. Early suspicion is critical as up to 65% of fatalities occur within 1 hour of the onset of a PE symptom.24
2. Venous thromboembolism morbidity. There is also significant morbidity associated with VTE such as pulmonary hypertension, venous insufficiency, and postthrombotic syndrome.19
B. Pregnancy effects on venous thromboembolism
1. Venous thromboembolism risk. The risk of VTE is greatly increased during pregnancy with an estimated 5 to 10 times greater incidence than outside of pregnancy.21,25,26
2. Venous thromboembolism timing. VTE can occur at any time during gestation, although the incidence increases in the third trimester and peaks following delivery, declining to prepregnancy levels within 12 weeks. The highest risk may be in the first week postpartum.18 The overall risk is at least 20 times greater in the first 6 weeks postpartum than in the nongravid state.18,19,25
3. Venous thromboembolism and hematologic changes in pregnancy. Pregnancy increases the risk of PE due to a combination of factors, such as hypercoagulability, hemodynamic changes, and endothelial injury. During pregnancy, there is an increase in venous stasis, increased production of coagulation factors including fibrinogen and von Willebrand factor (vWF), decreased production of inhibitors of coagulation, and a suppressed fibrinolytic state.27
CLINICAL PEARLHematologic changes in pregnancy contribute to the risk of VTE. These include higher levels of fibrinogen; vWF; and factors V, VII, VIII, IX, X, and XII. Additionally, the anticoagulant activity of protein S is decreased and activated protein C resistance increases. Thrombolysis is decreased as a result of increased activity of plasminogen activator inhibitor types 1 and 2 and decreased activity of tissue plasminogen activator. Early suspicion for VTE is critical because as many as 65% of fatalities occur within 1 hour of the onset of a PE symptom.
C. Symptoms of venous thromboembolism and pulmonary embolism
1. Similarity of pregnancy and pulmonary embolism symptoms. The diagnosis of PE requires a high index of suspicion because the symptoms of PE resemble the normal changes of pregnancy, such as shortness of breath, dyspnea on exertion, and a slightly increased heart rate. For this reason, the diagnosis of PE is frequently missed in parturients. If one suspects PE, it is important to evaluate and treat promptly.20
2. Venous thromboembolism symptoms. The symptoms of DVT are leg pain and redness. During pregnancy, the left leg and the proximal deep veins (iliac and femoral) are most commonly involved.26 Symptoms of PE may include dyspnea, palpitations, anxiety, cough, and chest pain. Common presenting signs include tachypnea, respiratory rales, and tachycardia. A large acute PE can increase pulmonary artery pressure, leading to right ventricular failure, decreased cardiac output, increased ventilation/perfusion (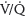 ) mismatch, and arterial hypoxemia. This could result in cardiovascular and respiratory collapse with syncope, hypotension, pulseless electrical activity, and death.26
) mismatch, and arterial hypoxemia. This could result in cardiovascular and respiratory collapse with syncope, hypotension, pulseless electrical activity, and death.26
D. Risk factors for venous thromboembolism. There are many risk factors, in addition to pregnancy itself, which increase the incidence of PE. It is important to offer prophylactic therapy to patients at increased risk. Risk factors for VTE development include a history of a previous DVT, thrombophilias (deficiencies of antithrombin and proteins C and S, factor V Leiden, and prothrombin gene mutation), the presence of a lupus anticoagulant and anticardiolipin/β2 glycoprotein 1 antibodies, obesity, CD, surgical procedures during pregnancy, and advanced maternal age.20,26
E. Imaging studies. A combination of lower limb compression Doppler ultrasonography (CUS), chest X-ray (CXR), nuclear  scanning, and computed tomography pulmonary angiogram (CTPA) is used to make the diagnosis of PE. The order of studies varies among practitioners. If there is a high clinical suspicion, and/or a positive CUS or high-probability
scanning, and computed tomography pulmonary angiogram (CTPA) is used to make the diagnosis of PE. The order of studies varies among practitioners. If there is a high clinical suspicion, and/or a positive CUS or high-probability  scan or CTPA, treatment should begin or continue.
scan or CTPA, treatment should begin or continue.
1. Ultrasonography. This can diagnose a DVT without any radiation exposure and allows prompt treatment if positive. It has a 97% sensitivity and 94% specificity for a lower extremity DVT, but is less reliable for pelvic DVTs.26 A proximal thrombus is found in only 23% to 52% of pregnant women with confirmed PE.26
2. Chest x-ray. If the CUS is negative, a CXR, which confers negligible risk to the fetus, may help identify other etiologies or reveal pulmonary edema, effusions, focal opacities, and atelectasis.19,28 Frequently, the CXR is normal (50%) and further workup will be required.26 If there is an abnormality on CXR, a CTPA is done. If the CXR is normal, either a CTPA or 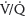 scan can be performed.
scan can be performed.
3. Ventilation/perfusion scan versus computed tomography pulmonary angiogram. The decision to proceed with either a 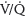 scan or CTPA is controversial.28
scan or CTPA is controversial.28
a. Ventilation/perfusion scans. Some practitioners prefer 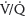 scans, especially after a normal CXR, because of the high negative predictive value, infrequent comorbid pulmonary disease in parturients, and lower maternal radiation exposure.19 However,
scans, especially after a normal CXR, because of the high negative predictive value, infrequent comorbid pulmonary disease in parturients, and lower maternal radiation exposure.19 However, 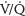 scanning in pregnancy is inconclusive in 25% of patients, which might necessitate further testing and radiation exposure.29
scanning in pregnancy is inconclusive in 25% of patients, which might necessitate further testing and radiation exposure.29
b. Computed tomography pulmonary angiogram. CTPA delivers less radiation to the fetus than 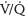 scanning and has a decreased lifetime cancer risk than
scanning and has a decreased lifetime cancer risk than 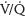 scanning (<1:1 million vs. 1:280,000, respectively). However, CTPA delivers higher doses of radiation to maternal breast tissue and increases the risk of breast cancer by as much as 14%.19–21,26 Nonetheless, CTPA allows visualization of the lung, mediastinum, and chest wall such that other etiologies can be excluded. CTPA is the preferred test for unstable patients and has a better sensitivity and specificity than a
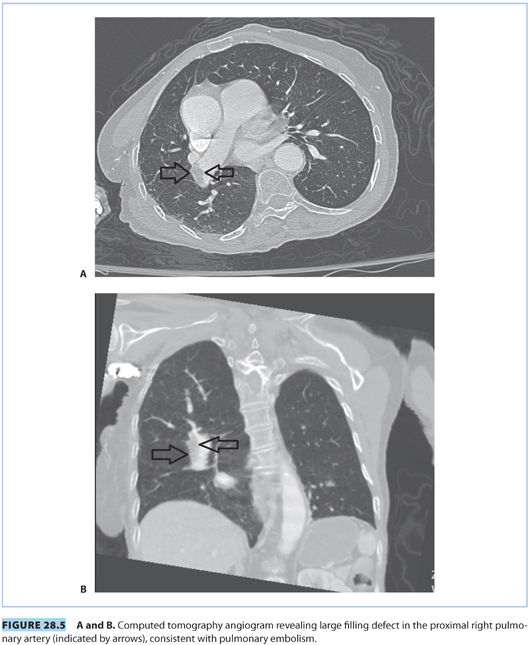
scanning (<1:1 million vs. 1:280,000, respectively). However, CTPA delivers higher doses of radiation to maternal breast tissue and increases the risk of breast cancer by as much as 14%.19–21,26 Nonetheless, CTPA allows visualization of the lung, mediastinum, and chest wall such that other etiologies can be excluded. CTPA is the preferred test for unstable patients and has a better sensitivity and specificity than a 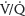 scan and fewer undergo further scans. If nuclear testing is positive, treatment should be continued. Nondiagnostic nuclear imaging will require workup with pulmonary angiogram/serial CUS and magnetic resonance imaging (MRI) studies.20 See Figure 28.5 for PE demonstrated on CTPA.
scan and fewer undergo further scans. If nuclear testing is positive, treatment should be continued. Nondiagnostic nuclear imaging will require workup with pulmonary angiogram/serial CUS and magnetic resonance imaging (MRI) studies.20 See Figure 28.5 for PE demonstrated on CTPA.
4. Ventilation/perfusion single photon emission computed tomography. The ventilation/perfusion single photon emission computed tomography ( SPECT) is a new three-dimensional imaging modality that has gained popularity and has a higher sensitivity and specificity compared to planar
SPECT) is a new three-dimensional imaging modality that has gained popularity and has a higher sensitivity and specificity compared to planar  scanning (97% and 91% compared to 76% and 85%, respectively). In addition, the radiation dose is about 35% to 40% less than CTPA, with much less radiation to maternal breast tissue.21
scanning (97% and 91% compared to 76% and 85%, respectively). In addition, the radiation dose is about 35% to 40% less than CTPA, with much less radiation to maternal breast tissue.21
5. Echocardiography. Echocardiography helps to visualize a massive centrally located PE, particularly in patients who are hemodynamically unstable. In addition, there could be signs of pressure overload of the right ventricle (RV), such as RV dilation and hypokinesis, tricuspid regurgitation, pulmonary hypertension, flattening and paradoxical motion of the septum, and lack of inspiratory collapse of the inferior vena cava.26,28
6. D-dimer. D-dimers indicate recent thrombus formation; they are plasma breakdown products of cross-linked fibrin. D-dimers increase in pregnancy, and by the third trimester, very few parturients will have a negative D-dimer. However, a low level would be suggestive that there is no PE. (i.e., has good negative predictive value).20
F. Treatment. Typically, patients who are not pregnant are treated with low molecular weight heparin (LMWH) in the acute period followed by chronic warfarin therapy. Warfarin cannot be used in pregnancy because it crosses the placenta leading to an embryopathy early in pregnancy and has been associated with intraventricular hemorrhage and schizencephaly later in pregnancy.21 LMWH and unfractionated heparin (UFH) do not cross the placenta. Further benefits include weight-based dosing, lack of need for monitoring blood values, decreased risk of thrombocytopenia, and decreased risk of osteoporotic fractures compared to UFH. UFH used to be the standard of care for acute embolism, but this has changed to LMWH for the treatment of DVT and PE.20,25
CLINICAL PEARLLMWH does not cross the placenta. Warfarin is contraindicated in pregnancy. Warfarin early in pregnancy causes an embryopathy. Later in pregnancy, warfarin increases the risk of fetal intracranial bleeding, stillbirth, ventricular septal defect (VSD), and growth retardation.
1. Optimum dosing. The physiologic changes of pregnancy, such as increased renal clearance and plasma volume, lead to a shorter half-life of LMWH. Further testing to determine the optimum dosing during pregnancy for the treatment of DVT/PE may be needed.21
2. Unfractionated heparin. UFH is used for life-threatening PE treatment.
3. Thrombolysis. In patients with hemodynamic compromise, thrombolysis may be beneficial in decreasing the clot burden and improving hemodynamics. There is an increased risk of maternal bleeding and fetal loss. Some fibrinolytics such as streptokinase do not cross the placenta.26,28
4. Embolectomy. If a patient remains hemodynamically unstable after medical management, emergency thoracotomy with embolectomy has been lifesaving.22
5. Extracorporeal membrane oxygenation. In patients with a massive PE resulting in RV failure and hypoxemia, extracorporeal membrane oxygenation (ECMO) has been used as a bridge to embolectomy, or to stabilize the patient so that she can benefit from anticoagulation.
6. Vena cava filter. Experience with venacaval filters in pregnancy is limited, but they may be helpful if delivery is eminent.20
G. Anesthetic implications of venous thromboembolism treatment. Closer to delivery, most practitioners switch from LMWH to subcutaneous (SQ) heparin in order to avoid bleeding, and so that patients can benefit from neuraxial analgesia and anesthesia. This typically occurs at 36 weeks’ gestation.
1. Therapeutic low molecular weight heparin and neuraxial analgesia. If a patient on LMWH is in labor, no further LMWH should be administered. Typically, epidural analgesia and spinal anesthesia can be performed 24 hours after therapeutic doses of LMWH. The LMWH should not be restarted until at least 2 hours after epidural catheter removal. However, if a patient undergoes a surgical procedure, then LMWH should not be administered regardless of anesthetic technique for 24 hours. If blood is encountered during neuraxial placement, the administration of LMWH should be delayed for 24 hours postoperatively.30
2. Prophylactic low molecular weight heparin and neuraxial analgesia. If the patient is on once-a-day prophylactic dose of LMWH, neuraxial anesthesia and analgesia can be administered 12 hours after the last dose. LMWH dosing should not resume for 2 hours after catheter removal. However, removal should occur at least 12 hours after the last dose of LMWH. After a surgical procedure in patients on prophylactic therapy, LMWH use should wait for 6 to 8 hours.30
3. Unfractionated heparin. Recommendations on IV UFH are lacking. Typically, UFH is discontinued and laboratory testing done to confirm a normal coagulation profile. SQ UFH in prophylactic doses does not contraindicate neuraxial analgesia or anesthesia. Higher doses should prompt laboratory investigation before placing a block.30
4. Neurologic checks. Even when you follow the recommended guidelines, the patient should be observed postpartum for signs of epidural hematoma.
CLINICAL PEARLNeuraxial analgesia and anesthesia should not be administered until 24 hours after the last dose of therapeutic LMWH, 1.5 mg per kg daily or 1 mg per kg twice a day. Neuraxial analgesia and anesthesia can be administered 12 hours after therapeutic dosing, usually 40 mg SQ daily. LMWH should not be given before 2 hours after catheter removal, assuming that the last dose was 12 or 24 hours prior to catheter removal for prophylactic and therapeutic dosing respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







