Critical illness is a catabolic state and critically ill patients are often malnourished. This chapter begins with a discussion of the pathophysiologic processes of malnutrition.
 Delivery of proper nutrition begins with accurate patient assessment. Nutritional assessment tools for critically ill patients are covered in the chapter.
Delivery of proper nutrition begins with accurate patient assessment. Nutritional assessment tools for critically ill patients are covered in the chapter.
 To aid in optimal nutritional construction, the estimation of energy requirements, protein requirements, and nitrogen balance is discussed.
To aid in optimal nutritional construction, the estimation of energy requirements, protein requirements, and nitrogen balance is discussed.
 To finish the construction of a nutritional support, the chapter contains a discussion of macronutrients and micronutrients.
To finish the construction of a nutritional support, the chapter contains a discussion of macronutrients and micronutrients.
 Most efficient nutrition occurs with enteral feeding, when possible. The timing and strategies for initiation of enteral feeding are covered in the chapter.
Most efficient nutrition occurs with enteral feeding, when possible. The timing and strategies for initiation of enteral feeding are covered in the chapter.
 For patients who cannot receive enteral nutrition, parenteral feeding strategies, monitoring, and complications are also discussed.
For patients who cannot receive enteral nutrition, parenteral feeding strategies, monitoring, and complications are also discussed.
The goals of nutritional support are to provide protein and caloric replacement while attenuating a negative nitrogen balance. It is estimated that 50% of hospitalized patients and 60% to 80% of ICU patients are malnourished. Delays in the initiation of nutritional support may result in muscle atrophy, failure to wean from the ventilator, gastrointestinal (GI) atrophy, heart failure, impaired immunity, increased incidence of sepsis, increased hospital length of stay, and increased morbidity and mortality.
There is no doubt that effective nutritional support with appropriate monitoring improves patient outcomes. Since the early 1980s, the importance of nutritional support in the patient who has sustained severe traumatic brain injury has been appreciated, but recent studies have demonstrated the same nutritional and metabolic requirements in patients who have acute ischemic damage and other critical neurologic illnesses. There is also compelling evidence that strict glycemic control in the critically ill patient reduces morbidity and mortality.
I. PATHOPHYSIOLOGY
The effects of elective operations, trauma, and critical illness activate the neural and endocrine systems. The increase in sympathetic outflow leads to lipolysis, proteolysis, and decreased glucose uptake from the antagonism of insulin by growth hormone and epinephrine. This sympathetic surge acts to increase energy expenditure (EE), tissue catabolism, and mobilization of protein, fat, and carbohydrate. The deleterious effects of augmented sympathetic outflow are exacerbated by immobility and delays in the initiation of nutritional support. Other adverse effects include hyperglycemia, poor wound healing, decreased serum proteins, increased carbon dioxide (CO2) production, release of inflammatory mediators, and depressed immune function
A. After neurologic injury, the ensuing state of cardiac hyperdynamism increases oxygen consumption and caloric requirements. Studies using indirect calorimetry demonstrate that severely head-injured patients are hyper-metabolic and hypercatabolic. Resting energy expenditure (REE) can increase by as much as 40% to 165%. Patients demonstrate this increase in REE regardless of whether or not they have received steroids. In addition, they can also experience delayed gastric emptying, bacterial translocation, and altered vascular permeability with resultant intestinal edema and malabsorption.
1. Patients who have sustained head injury exhibit changes consistent with hypermetabolism, hypercatabolism, hyperglycemia, suppressed immunity, and generalized inflammatory response. Nutritional support should satisfy these physiologic demands.
2. Patients who have suffered a stroke also exhibit hypermetabolism. Although it has been suggested that protein supplementation beyond normal nutritional support after stroke may improve outcome, recent trials have not supported this strategy.
B. Patients who are obese, and recent estimates indicate that approximately 40% of adults in the United States are overweight, are more likely to have comorbid medical conditions including hypertension, coronary artery disease, diabetes mellitus, pulmonary disease, and gout. Critical illness is likely to result in protein malnutrition, inhibition of lipolysis, and preferential use of carbohydrate substrate for gluconeogenesis. It has been suggested that patients who have actual body weights (ABWs) in excess of 125% of ideal body weight (IBW) may benefit from diets that are hypocaloric and high-protein in structure. The goal for obese patients is to provide adequate protein intake to meet and not exceed metabolic demands. Ideally, nutritional supplementation contains enough protein to keep the patient in a positive nitrogen balance, but a sufficient calorie deficit to encourage net increase in lean body mass (LBM) and loss of total body fat.
II. NUTRITIONAL ASSESSMENT
The delivery of appropriate metabolic support begins with an assessment of the patient’s nutritional status. It is important to identify patients who present with subtle signs of deficiencies in caloric, protein, vitamin, or trace metal intake. These patients should be considered for earlier and more aggressive nutritional support.
A. Definitions. The malnourished state exists when intake does not meet nutritional demands. When approximately 10% of LBM is lost, moderate protein malnutrition exists. A loss of 20% of LBM indicates severe protein malnutrition and describes a severely underweight patient. When > 35% of LBM is lost, it is likely that irreversible changes leading to death have occurred.
B. History. In most cases, a history of decreased spontaneous food intake and unintentional weight loss (5% of LBM in 1 month or 10% of LBM in 6 months) precedes admission to the intensive care unit (ICU). A careful social history may demonstrate vitamin or mineral deficiencies associated with alcoholism or other substance abuse. Patients who have renal failure lose amino acids, vitamins, and trace metals during dialysis. Patients who have cancer may have deficiencies owing either to the underlying disease or to chemotherapy (e.g., methotrexate). Other risk factors for malnutrition include age > 75 years, homelessness, nothing by mouth for more than 3 days, major surgery, corticosteroid administration, malabsorption syndromes, and chronic disease states, especially cancer, stroke, and acquired immunodeficiency disease.
C. Physical examination. Caloric intake can be assessed by the amount of fat in the extremities, buttocks, and buccal fat pad. The adequacy of protein intake can be evaluated from the bulk of the extremity muscles, grip strength, and the size of the temporal muscle. Vitamin deficiencies may manifest themselves as changes in skin texture and hair quality and texture, cheilosis, glossitis, or loss of vibration and position sense. Examination of the head and neck may reveal xerostomia, malocclusion, odynophagia, dysphagia, esophagitis, or other findings suggestive of difficulty with eating.
D. Anthropometrics. Measurements are used to estimate the stores of body fat and protein. Body fat is approximated by the thickness of the triceps skin fold (TSF) and protein status is estimated by the midarm muscle circumference (MAMC).
MAMC = midarm circumference – fat
MAMC = midarm circumference – (0.314 × TSF)
These data are then compared with normal values to determine the patient’s nutritional status. Two possibly incorrect assumptions are that body fat is uniformly distributed and that population standards apply to patients who are critically ill. In fact, anthropometric measurements are generally invalid in the critically ill owing to anasarca. Adjustments in estimations can be based on IBW calculated from the measurements of height and weight.
E. Biochemical measurements. A variety of biochemical and metabolic measurements have been studied as potential indices of nutritional status. Although many of these tests have significant value in assessing stable patients who are scheduled for elective surgery and those well on the way to recovery, their applicability to critically ill patients has not been demonstrated. The same patient’s measurements taken on different days can be compared only if other factors are relatively stable.
1. Plasma proteins. The level of plasma protein depends on the synthetic function of the liver and the availability of substrate. Unfortunately, a decrease in plasma protein levels is not specific because the biologic half-life, the catabolic rate, and a variety of non-nutritional factors can alter them. For example, expansion of the extracellular fluid compartment results in a reduction in albumin concentration. In addition, the levels of some serum proteins decrease promptly in response to trauma, sepsis, or severe illness because of fluid shifts, alterations in capillary permeability, and changes in the rates of synthesis and degradation. Liver disease, nephrotic syndrome, eclampsia, and protein-losing enteropathies are additional causes of hypoproteinemia.
a. Albumin has a half-life of approximately 18 days. Its measurement is the most commonly used test to diagnose protein–calorie undernutrition. Depending on measured albumin alone, however, can lead to delays in treating nutritional deficits because plasma levels can be maintained for quite a while owing to the long half-life. In critical illness, albumin may also remain low until the remission of the inflammatory response despite adequate nutritional intake. Serum albumin levels have been shown to be good predictors of sepsis and major infection in surgical patients. However, serum albumin levels have not been correlated with either morbidity or mortality in the setting of severe neurologic injury.
b. Transferrin has a half-life of approximately 8 days. Therefore, transferrin levels are a more accurate reflection of acute changes in nutritional status. Low serum transferrin is indicative of protein malnutrition. Iron deficiency, pregnancy, and hypoxia stimulate synthesis of transferrin whereas chronic infection, sepsis, and iron overload decrease transferrin levels.
c. Thyroxine-binding prealbumin and retinol-binding protein (RBP) have a half-life of 2 to 3 days and 8 to 12 hours, respectively. RBP complexes with prealbumin in the circulation. The resultant compounds are much more sensitive to short-term alterations in protein and total calorie intake; low levels can return to normal after only 3 days of adequate nutritional support. While serum levels of the RBP–prealbumin complex reflect acute changes in nutritional status and indicate the adequacy of nutritional supplementation, many non-nutritional factors can affect these levels as well. Infection and trauma depress prealbumin levels. Stress and vitamin A deficiency decrease the concentration of RBP; renal failure can cause erroneous elevation of RBP. The short half-lives and the myriad of factors influencing their serum concentrations have limited the clinical usefulness of these proteins.
2. Immunologic functions. Total lymphocyte count and reactivity to skin test antigens are immunologic functions that reflect nutritional status, but they are not routinely used in patients who are critically ill. Any stressful situation, especially any disease process that requires a stay in the ICU, can depress cellular immunity, leading to a non-specific test result.
III. ESTIMATION OF ENERGY REQUIREMENTS
The previously mentioned tests aid in assessing nutritional status. Different methods are needed, however, to estimate a patient’s ongoing nutritional and caloric needs based on his or her individual disease process and body habitus. This is accomplished by using predictive equations or indirect calorimetry.
A. Predictive equations can be used to estimate the REE, a patient’s energy requirement at rest. More than 200 different equations have been published, many of which are variations of the Harris-Benedict equation.
Women: REE (kcal/day) = 65 + 9.6W + 1.8H – 4.7A
Men : REE (kcal / day) = 66 + 13.7W + 5H – 6.8A
where W is the ABW in kilograms or IBW in kilograms if the patient is edematous; H is the height in centimeters, and A is the age in years. If the patient is obese, an adjusted IBW should be used as follows:
Adjusted IBW = IBW + 0.25 (ABW – IBW).
Using predictive equations such as the Harris-Benedict equation, which was developed using healthy volunteer subjects, has many limitations and the validity of its application in the critically ill has been questioned. Therefore, some clinicians actually prefer to use a simplified weight-based formula: 25 to 30 kcal/kg/day.
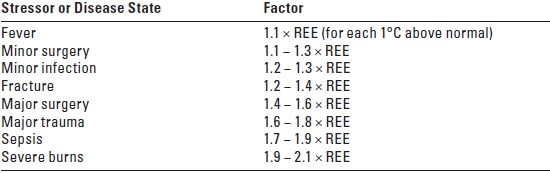
1. Adjustments to REE. The EE of patients can vary greatly from the daily REE in active subjects. Hospitalized patients typically require 30 kcal/ kg/day in the absence of severe illness or obesity. Fever increases the REE by approximately 10% above baseline for each degree Celsius rise in temperature. Brain injury induces a hyperdynamic state. An elevated EE is also associated with total body surface area burns of 20% to 40%.
2. The estimated REE is adjusted for the state of hypermetabolism that characterizes patients who are critically ill. Table 25.1 shows the usual correction factors that are used.
TABLE 25.1 Correction Factors for the Adjustment of REE for Several Stressors and Disease States
B. Indirect calorimetry provides a more accurate measure of EE than predictive equations, especially in patients who are obese. It relies on the measurement of oxygen consumption (VO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






