Nutritional Assessment and Support
1 For patients with preexisting nutritional deficiencies, profound hypermetabolic states, or prolonged ICU stays, nutritional support is probably important. By contrast, for patients with normal nutritional status and brief ICU stays, nutrition is much less critical.
2 Detailed nutritional assessments and metabolic cart studies are expensive and offer little benefit for most ICU patients.
3 Enteral feeding delivered to the stomach using a small-bore nasal or oral tube is preferred over total parenteral nutrition in essentially all circumstances where the gut is functioning.
4 Both enteral and parenteral nutrition are associated with significant complications: aspiration with the former and metabolic and septic complications with the latter.
5 If intravenous nutritional support is required, central total parenteral nutrition delivered through a freshly placed dedicated catheter is the best option. The total parenteral nutrition prescription should be customized to meet patient requirements and limitations based on underlying organ failures.
Perhaps even more so than ventilator or hemodynamic management, critical care nutrition is controversial. Discussions are polarizing with strong opinions held about when, what, how, and how much support should be provided. Unfortunately, the controversies are yet to be resolved by large, randomized, controlled clinical trials, so much of current practice defaults to habit and belief. However, four basic facts are rarely disputed: malnutrition is associated with poor outcomes, starvation for weeks to months is fatal, several days without food is well tolerated by previously healthy humans, and enteral or parenteral nutrition support is far different than eating a regular diet.
▪ WHY FEED CRITICALLY ILL PATIENTS?
In the intensive care unit (ICU), pressing concerns of hemodynamic and respiratory instability often divert attention away from nutrition. Yet, by impairing immunity, prolonging ventilator weaning, and delaying wound healing, malnutrition could prove decisive in the fragile patient with a long stay. However, most patients stay only a few days in the ICU, which raises the question of how important it is to begin support rapidly or to achieve full caloric goals. Despite the inherent appeal of providing nutrition, there is surprisingly little credible evidence that nutritional support improves important clinical outcomes among the critically ill, and there are significant complications associated with “feeding,” especially after long periods of starvation. Even though a convincing survival benefit has not been demonstrated for either enteral or parenteral support, studies demonstrating improvement in surrogate markers of nutritional status (e.g., serum protein concentrations or lymphocyte counts) and length of stay or infectious complications and the visceral appeal of “feeding” have promoted an entire industry.
▪ WHY WITHHOLD NUTRITION SUPPORT?
Historically, many physicians and nurses have assumed the gastrointestinal (GI) tract is so dysfunctional that enteral nutrition in the critically ill patient was not feasible. Given this belief only two options existed: begin parenteral nutrition or withhold all support. Thus, many patients were not given nutrition because of the risks, costs, and
complexity of the parenteral route. However, the realization has come over time that there are advantages of enteral nutrition over parenteral nutrition and that enteral feeding is much more practical than once believed. Furthermore, it is likely that some catabolic patients, especially those with severe sepsis, ineffectively utilize nutrients in any form. Even if nutritional substrate could be fully used by the tissues of seriously ill patients, the high likelihood of survival and brief stay of most ICU patients raises serious doubt about the ability of any form of nutritional support to improve survival or shorten length of stay (or at least to demonstrate such benefit in a clinical trial). Despite widespread advocacy for early and full feeding, studies of current practice indicate most critical care physicians are content to delay the institution of nutritional support for days. Expert consensus statements suggest that even a week of delay is acceptable. In addition, several small studies suggest that early full enteral nutrition may be associated with worse outcomes; perhaps due to enhanced inflammation. Once a decision has been made to provide supplemental nutrition, three basic questions need to be answered: (i) What route of feeding will be used? (ii) How many calories and how much protein will be delivered? and (iii) Are there any special considerations related to the patient’s underlying diseases?
complexity of the parenteral route. However, the realization has come over time that there are advantages of enteral nutrition over parenteral nutrition and that enteral feeding is much more practical than once believed. Furthermore, it is likely that some catabolic patients, especially those with severe sepsis, ineffectively utilize nutrients in any form. Even if nutritional substrate could be fully used by the tissues of seriously ill patients, the high likelihood of survival and brief stay of most ICU patients raises serious doubt about the ability of any form of nutritional support to improve survival or shorten length of stay (or at least to demonstrate such benefit in a clinical trial). Despite widespread advocacy for early and full feeding, studies of current practice indicate most critical care physicians are content to delay the institution of nutritional support for days. Expert consensus statements suggest that even a week of delay is acceptable. In addition, several small studies suggest that early full enteral nutrition may be associated with worse outcomes; perhaps due to enhanced inflammation. Once a decision has been made to provide supplemental nutrition, three basic questions need to be answered: (i) What route of feeding will be used? (ii) How many calories and how much protein will be delivered? and (iii) Are there any special considerations related to the patient’s underlying diseases?
▪ SELECTING CANDIDATES FOR NUTRITION SUPPORT
Most previously healthy patients readily tolerate calorie deprivation for a week or more before supplementation is necessary. Although benefit is unproven, candidates likely to benefit from nutritional supplementation include (i) those unable to eat for long periods because of endotracheal intubation or GI tract interruption; (ii) patients with high caloric requirements (e.g., burns, severe sepsis, major surgery, or trauma); (iii) patients who sustain high protein losses (e.g., corticosteroid or tetracycline usage, nephrotic syndrome, or draining fistulas); and (iv) patients already malnourished at the time of ICU admission.
Complex nutritional assessment scales have been developed that incorporate anthropomorphic measurements and laboratory studies. Whereas such precise indices of nutritional status may occasionally be helpful, simple clinical evaluation—a history of weight loss, dietary habits, and knowledge of underlying disease—provides a good working assessment. Of the widely available clinical measures of nutritional status, clinical history, absolute lymphocyte count, cholesterol, and serum protein levels at the time of admission are perhaps the most useful data.
Malnutrition is almost certain in patients losing 5% of their body weight in the past month or more than 10% in the 6 months preceding admission. Absolute lymphocyte counts less than 1,200 cells/mm3 and less than 800/mm3 signify moderate and severe malnutrition, respectively. It is widely believed that because albumin normally has a long half-life (approx. 18 days), weeks of nutritional deficiency is needed to produce hypoalbuminemia. This is not true in the ICU population because vascular permeability for albumin is often dramatically increased; the liver ceases to produce albumin during severe illness; and extracellular water is often dramatically expanded. The net effect of these processes is to cause hypoalbuminemia within hours to days. Even faster declines may occur during intense catabolic stress. Transferrin and thyroid-binding globulin (TBG) prealbumin may be more sensitive acute indicators of response to nutritional depletion or therapy because they have shorter half-lives. In the absence of hypothyroidism, severe liver disease, or nephrotic syndrome and profound depressions of serum cholesterol reliably indicate calorie deprivation. Fever, severe sepsis, steroids, tumors, and immunosuppressant drugs reduce the value of the antigenic skin test response to the point of making it not clinically useful.
▪ NUTRITIONAL REQUIREMENTS
Energy/Calories
Formulas for calculating calorie requirements (basal energy expenditure [BEE]) are often complicated, as exemplified by the Harris-Benedict equation:
BEE (men) = 66 + (13.7 × wt) + (5 × ht) − (6.8 × age)
BEE (women) = 655 + (9.6 × wt) + (1.8 × ht) − (4.7 × age)
Weight (wt) and height (ht) are expressed in kilograms and centimeters, respectively, and BEE is kcal/day.
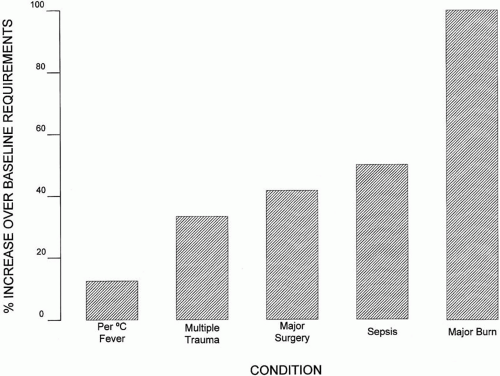
Despite their complexity and seeming precision, the Harris-Benedict formulas still require upward adjustment for additional stress. For example, a well-nourished, minimally active patient should receive 1.25 times the BEE, whereas a severely anabolic patient may expend up to 1.75 times the BEE. Although detailed calculations may occasionally prove helpful, adequate caloric requirements can usually be estimated by simple assessment of the patient’s general condition and lean body weight. Rather than calculating the Harris-Benedict equations, simply providing 25 to 35 kcal/kg/day will be close to the mark for most patients. In the severely stressed patient, two to three times that number of calories may be required (Fig. 16-1).
Bedside indirect calorimetry (metabolic cart study) provides a direct measure of caloric expenditure during the period the test is performed (usually 15 to 20 min). Resting energy expenditure (REE) is determined using the Weir equation (see following) by measuring oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (Ve). Accuracy of REE determinations are highly dependent on proper setup and calibration of the measuring device and are less reliable in patients receiving greater than 50% oxygen. Furthermore, calorimetry disrupts the patient care routine, is expensive, and is not universally available. Calorimetry is not necessary for most patients. REE measurements might be helpful in difficult-to-wean patients who may be overfed and in obese or edematous patients in whom current body weight may not provide an accurate estimation of caloric requirements.
Daily REE = [(VO2) (3.94) + (VCO2) (1.11)] × 1,440
(VO2 and VCO2 are in L/min, and REE is in kcal/day.)
About 80% of all calories should be supplied by nonprotein sources. Typically, glucose provides one half to three quarters of this (2 to 4 gm/kg/day), with the remainder provided as lipid. The exact proportions of glucose and lipid are not of crucial importance. However, at least 100 to 150 nonprotein kcal/gm of nitrogen (approx. 20 cal/gm protein) must be provided to avoid using amino acids as an energy source. Some glucose is required for protein-sparing effects and to supply those tissues with an obligate requirement for glucose (e.g., brain). However, when given in excess of 7 mg/kg/min, glucose is largely converted to fat, leading to potential complications of overfeeding (volume overload, hyperglycemia, increased CO2 production, and fatty liver).
Protein
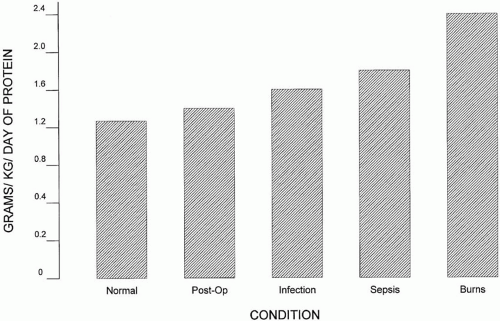
Normal adults require 0.5 to 1.0 gm/kg/day of protein; however, the average ICU patient may need up to 1.5 to 2.0 gm/kg/day (Fig. 16-2). The corresponding amount of nitrogen supplied may be calculated by dividing grams of protein by 6.25 (about 0.15 gm of N2/kg/day). Nutritional supplements provide amino acids capable of being reassembled intracellularly to form structural proteins and enzymes. Providing greater quantities of amino acids than required is not likely to be beneficial. (Excess amino acids cannot be stored; therefore overfeeding requires the oxidation and excretion of these compounds as urinary nitrogen wastes.) For most patients, anabolism or proteinsparing effects are not enhanced by administration of protein in amounts above 1.5 gm/kg/day. In addition to the normal routes of catabolism, important protein losses may also occur through the urine (in nephrotic syndrome), surgical drains, or chest tubes (especially chylothorax). The adequacy of protein delivery can be assessed using a urinary nitrogen balance study (detailed following); however, in practice, such studies are laborious and expensive and hence, are uncommonly performed.
The classical distinction between “essential” and “nonessential” amino acids is artificial. Histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine have been considered to be essential, but for critically ill patients all amino acids are probably “conditionally essential.” (Glutamine, alanine, and aspartate are the only nonessential amino acids, but now some data even suggests benefits of supplementing glutamine.)
Lipids
Some lipid intake is required to prevent the occurrence (over several weeks) of essential (linoleic) fatty acid deficiency. Minimal daily linoleic acid requirements are estimated to be between 3 and 20 gm, an amount easily supplied by providing as little as 5% of calories in the form of lipid. Usual lipid doses are in the range of 0.5 to 2.0 gm/kg/day. (In most patients, the limits of lipid metabolism are approximately 4 gm/kg/day.) Lipids also provide a rich source of calories in a small volume. This fact is supported by the observation that sedation using propofol with its lipid vehicle supplies 1 to 2 kcal/mL of drug infused. The lower respiratory quotient observed from the oxidation of fats as compared to carbohydrates indicates that fats generate a lower CO2 burden for any given caloric intake. Effective lipid metabolism requires a functioning liver; therefore, lipids may not represent the best caloric choice for patients with hepatic dysfunction.
Vitamins and Trace Elements
Vitamins and trace elements serve as antioxidants and play key roles as intracellular cofactors for enzymatic and energy generating reactions. More than a dozen different vitamins and trace minerals have been identified as essential for normal physiologic function. It is well recognized that levels of these substances are often abnormal in the plasma of critically ill patients.
TABLE 16-1 CLINICAL SYNDROMES RESULTING FROM VITAMIN DEFICIENCIES | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In general, fat-soluble vitamins (K, E, D, A) are less prone to acute changes induced by critical illness by virtue of their relatively large storage pool in most patients. Fat-soluble vitamin levels can be reduced in patients suffering from prolonged starvation or malabsorption and in patients treated with broad-spectrum antibiotics, warfarin compounds, or bile-sequestering drugs. By contrast, the water-soluble vitamins (C, folate, and other B complex vitamins) are prone to rapid declines when patients are subjected to dietary deprivation. (Vitamin B12 is an exception to this rule.) Table 16-1 provides a list of clinical conditions associated with specific vitamin deficiencies.
Individual deficiencies of the trace minerals, copper, zinc, selenium, chromium, manganese, and molybdenum, have all been associated with specific syndromes. A full discussion of these syndromes is well beyond the scope of this text. Suffice it to say that significant clinical deficiencies of these elements are rare given even meager nutritional support. Luckily, all commercially available tube feeding products and now essentially all parenteral nutrition solutions contain at least the daily minimum requirements of vitamins and trace minerals, making clinical deficiencies uncommon.
▪ THE ADVERSE EFFECTS OF MALNUTRITION
Malnutrition depresses the immune system by reducing immunoglobulin levels and by decreasing T cell function. Deficiencies of glutathione and other antioxidant compounds (vitamin E, β-carotene) may be associated with impaired resistance to the oxidative stress of sepsis, cancer chemotherapy, or high levels of inspired oxygen. Low colloid osmotic pressure caused by hypoalbuminemia predisposes to pulmonary and peripheral edema at a lower-than-normal hydrostatic pressure. Poor nutrition may impair wound healing and increase rates of infection in burns, trauma, and postoperative states. Starvation impairs ventilatory capacity by decreasing drive and diaphragm bulk. Severe nutritional deficiencies may be fatal, even in otherwise healthy patients. (Even under optimal conditions, otherwise healthy adults succumb to subacute losses greater than 40% of lean body mass.) When patients with prolonged starvation are refed, hypophosphatemia may cause muscle weakness.
▪ INDICES OF ADEQUATE SUPPORT
The same indices used to detect malnutrition may also be used to determine the effectiveness of nutritional supplementation. Because albumin is slowly
repleted, it is not a very useful index of acute improvement or deterioration in nutritional status. With much shorter half-lives, prealbumin and transferrin may be more helpful. Note that iron deficiency can increase transferrin levels independent of nutritional status. Weight gain is not a dependable sign; edema may produce rapid weight increases without improvement in nutritional status, and the average critically ill patient gains approximately 1 L of fluid each day.
repleted, it is not a very useful index of acute improvement or deterioration in nutritional status. With much shorter half-lives, prealbumin and transferrin may be more helpful. Note that iron deficiency can increase transferrin levels independent of nutritional status. Weight gain is not a dependable sign; edema may produce rapid weight increases without improvement in nutritional status, and the average critically ill patient gains approximately 1 L of fluid each day.
Nitrogen balance is possibly the best indicator of nutritional homeostasis. Because nonurinary (skin and stool) losses of nitrogen are usually small (approx. 2 gm/day), the 24-h urine collection effectively quantitates nitrogen losses. Urine urea nitrogen (UUN) accounts for 80% of total urinary N2 losses, and acute illness promotes urinary excretion of nitrogen as a result of catabolism. Hence, unless excessive nonurinary nitrogen losses occur (e.g., through fistulas or open surgical wounds), the balance between nitrogen intake and loss can be approximated using this formula:
N2 balance = (Protein intake/6.25) − ([UUN + 20%] + nonurinary losses)
▪ ROUTES OF NUTRITIONAL SUPPLEMENTATION
Enteral Therapy
The numerous advantages of enteral feeding almost always make it preferable to the parenteral route (Table 16-2). Enteral feeding is less expensive, deters GI ulceration, promotes immune function, and preserves small bowel mucosal integrity and function better than intravenous nutrition. Because enteral feeding stimulates insulin secretion, hyperglycemia is less likely than during total parenteral nutrition (TPN). Enteral feeding stimulated release of cholecystokinin and gastrin preserves normal emptying of the gallbladder and pancreatic secretions. Enteral feeding also provides iron and trace elements more effectively than TPN. Although the importance of any individual component found in enteral feedings is uncertain, nucleic acids, medium-and short-chain amino acids, fiber, glutamine, and intact proteins are not present in TPN solutions. The GI tract is often not used for nutrition in favor of TPN because of concerns that the gut is immotile. Because gastric peristalsis is often lost while small bowel function is preserved, air may not move into the small bowel to generate the characteristic gurgling bowel sounds in critically ill patients. Likewise, patients receiving continuous gastric suction or neuromuscular blocking drugs do not swallow air or fluid necessary to produce bowel sounds. Therefore, neither mild abdominal distention nor reduced bowel sounds should deter a trial of enteral feeding. In fact, it is often only after feeding is begun that bowel sounds return.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree