(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
Enteral nutritionTotal parenteral nutrition (TPN)Gastric residual volumeGastroparesisWheyCaseinProtein anabolismMyopathyBolus feedCarbohydratesOmega-3 fatty acidsStarvationMuscle wastingProteinObesityRefeeding syndromeThe traditional method of continuous tube feeding is illogical, stupid and quite likely harmful
Paul Marik MD, Intensivist, 1958–∞
During the last decade, the seemingly simple task of feeding critically ill patients has become exceedingly complex with much controversy [1]. While many consider nutrition support “an afterthought” current evidence suggests that in critically ill patients the approach to nutritional support directly impacts patient outcome. Furthermore, critically ill patients’ loose large amounts of lean body mass (muscle), and this has a dramatic effect on the ability of the patient to wean from mechanical ventilation and long term recovery. Emerging data suggests that a more rational approach to nutrition support (bolus feeding high quality protein) may limit the loss of lean body mass. In reality the approach to nutritional support in the ICU is quite simple (this is not rocket science):
Route—Enteral nutrition (EN)
PO (1st)
OG tube (2nd choice)
Post pyloric feeding tube (3rd choice)
Avoid TPN like the plague (no choice)
When
Within 24 h
Quantity
Day 1: 15 kcal/kg/day
Day 2: 20 kcal/kg/day
Day ≥ 3: 25 kcal/kg/day
Composition (tube feeds)
High quality protein, with branch chain amino acids especially leucine (Whey)
Omega-3 fatty acids and structured lipids
Low glycemic index
Soluble fiber
HOW
Bolus feeds
The two commandments of nutrition support
“If The Bowel Works, Use It” (and if it don’t work, make it work)
“There is no disease process that benefits from starvation”.
Myths of Nutritional Support [2]
Starvation is “okay”
TPN is safe
EN is contraindicated with vasopressors
EN contraindicated with mechanical ventilation
EN is contraindicated with high gastric residual volumes
Post-pyloric feeding reduces the risk of aspiration
EN is contraindicated in patients without bowel sounds and/or postoperative ileus
EN is contraindicated following GI surgery
EN is contraindicated with an open abdomen
EN is contraindicated in patients with pancreatitis
Patients must be fed semirecumbent at 45° The risk of aspiration is equivalent with the head of bed at 20° as compared to 45°
Bowel rest. In reality there is no such thing, the bowel never stops functioning and trying to “rest the bowel” is akin to inducing cardiac arrest to rest the heart.
All ICU patients should be fed soon after admission to the ICU. The only exceptions include:
Patients with GI bleed (GIB) who will require endoscopy. Feeding must be resumed after completion of endoscopy
Patients with ischemic bowel (this is a surgical problem that must be fixed)
Patients with bowel obstruction (this is a surgical problem that must be fixed)
Patients in whom extubation is planned within 12 h in whom oral feeding will be resumed
Patients who are about to die or are dead
The results of a number of RCT’s (and meta-analyses of these trials) have established the following principles on which to base nutritional support in critically ill patients [3–10]:
There is no data that parenteral nutrition (TPN) is of any benefit in critically ill patients. The available evidence suggests that TPN increases complications and mortality rates [2, 4]. TPN should not be given to supplement the “nutritional deficit” associated with enteral nutrition. PN should therefore be limited to patients, who after 5–7 days of starvation are unable to tolerate even small volumes of enteral nutrition. This includes patients with proximal small bowel fistula, unresolving bowel obstruction and short gut syndrome [11].
Early enteral nutrition (within 24 h of admission to the ICU) has been shown to reduce complications and improve the outcome of critically ill patients when compared to delayed enteral nutrition [12, 13]. The data is most convincing in postoperative patients. No studies demonstrate an advantage to delaying nutritional support in seriously ill patients.
In critically ill medical and trauma patients’ an initial strategy of permissive underfeeding has been demonstrated to be well tolerated and did not appear to compromise long term outcomes. It is important to note that these studies used continuous tube feeds; this is an outdated and potentially harmful feeding strategy that should be abandoned (see below).
Overfeeding patients is associated with significant complications including hyperglycemia, hepatic steatosis with hepatic dysfunction, elevated BUN and excessive CO2 production
In surgical patients an aggressive approach to peri-operative enteral nutrition including a rapid escalation in volume and the use of immunonutrition has been demonstrated to reduce postoperative complications [14].
There is no data to suggest that accurately measuring resting energy expenditure (REE) to determine nutritional requirements improves outcome. Measurement of REE may be appropriate in morbidly obese patients.
Enteral nutritional feeding formulas of low osmolarity and enriched with fibre (nondigestible plant cell wall constituents) reduce the risk of diarrhea and improve feeding tolerance.
Emerging experimental data suggests that administering enteral nutrition (with high quality protein) as intermittent boluses rather than as a continuous infusion results in a more physiological endocrine profile with greater protein synthesis (see below)
Whey protein results in greater protein anabolism than soy or casein protein (see below)
Important Points to Digest
A large number of different enteral nutrition formulations are available (see Table 32.1). While many hospitals have a complex list (with many tables) of formulations in reality only four different formations are required, namely
Table 32.1
Composition of common enteral formulas
Product
Type
Kcal/mL
Osmol.
CHO g/L (% cals)
Protein g/L (% cals)
Fat g/L (% cals)
Source of lipid
n-6:n3
Source protein
Added Arg. (g)
Manufacturer
Osmolite 1.2
St w/o fiber
1.2
360
158/53
56/18
39/29
Canola oil
6.3:1
Casein Soy
0
Abbott
MCT oil
Jevity 1.2
St w fiber
1.2
450
169/52
55/18
39/29
Canola oil
6.3:1
Casein Soy
0
Abbott
MCT oil
Glycerna 1.2
Diabetic
1.2
720
114/35
60/20
60/45
Canola oil
3.3:1
Casein Soy
0
Abbott
Fish oil
Vital AF 1.2
S-elemental
1.2
425
110/36
75/25
54/39
Fish, Soy
0.9:1
Whey
0
Abbott
Canola, MCT
Casein
Nephro
Fluid elect
1.8
745
161/34
81/18
96/48
Canola
5.2/1
Casein
0
Abbott
restricted
Safflower
Pivot
IMD
1.5
595
172/45
93/25
51/30
Fish, Soy
1.7:1
Whey
13
Abbott
Canola, MCT
Casein
Replete
St w fiber
1.0
319
113/45
62/25
34/30
Canola oil MCT oil
2.3:1
0
Nestle
Peptamen AF
S-elemental IMD
1.2
390
107/36
75/25
55/39
Fish, MCT, Soy
1.8:1
Whey
0
Nestle
Novosource Renal
Fluid elect
2.0
800
183/37
90/18
100/45
Canola, MCT
–
Soy
0
Nestle
restricted
Fibersource HN
St w fiber
1.2
490
160/53
53/18
39/29
Canola oil, MCT
2.7:1
Soy
0
Nestle
Vivonex Plus
Elemental low fat
1.0
650
188/76
45/18
6.6/6
Soy
7.0:1
Free amino acids
6.3
Nestle
Impact
S-elemental
1.0
375
132/53
56/22
28/25
Fish, sunflower, safflower
1.4:1
Casein
12.5
Nestle
IMD
a general all-purpose formula suitable for most patients (whey and omega 3)
a formulation with arginine and omega-3 fatty acids for peri-operative and trauma patients
a volume and electrolyte reduced formulation for patients with renal failure not receiving dialysis when a general all-purpose formula is not suitable
a low fat elemental formulation for patients on high dose propofol, the initial formulation for severe pancreatitis and patients with fistula, short gut syndrome, etc.
There is no specific generic all-purpose formulation which has been proven to improve outcome. However we use a formula with following characteristics:
In excess of 70 % of ICU patients develop stress hyperglycemia. Common sense would therefore dictate using a formulation higher in fat than carbohydrate (low glycemic index)
As most patients have some degree of inflammation we prefer that the fat content include omega-3 fatty acids
As many patients may have issues with lipid absorption and metabolism we prefer a structured lipid with medium chain triglycerides
We prefer a formulation that has whey rather than casein or soy as the major protein, as whey contains leucine which is an essential branch chain amino acid that is a prerequisite for protein synthesis
The formula should have a low osmolarity to reduce the risk of diarrhoea
The absence of bowel sounds does not mean that the bowel is not working. In the ICU patient population, neither the presence of bowel sounds nor evidence of the passage of flatus or stool is required for the initiation of enteral feeding [2].
There is a poor relationship between the gastric residual volume and the risk of aspiration [15–17]. DO NOT hold tube feeds unless the gastric residual volume >400 mL and/or the patient shows signs of intolerance (distended abdomen, vomiting)
In patient’s receiving continuous tube feeds hypoglycemia may occur when tube feeds are suddenly discontinued. The patient should be placed on a D5 or D10 solution (to prevent hypoglycemia) and the blood glucose monitored closely (particularly when the patient is on an insulin protocol). However continuous tube feeding is not recommended (see below)
Making the patient nil per os (NPO) surrounding the time of diagnostic tests or procedures should be minimized to prevent inadequate delivery of nutrients and prolonged periods of ileus. Ileus may be propagated by NPO status. If the patient is intubated there no need for tube feeds to be stopped hours before a procedure. This issue becomes less of a problem with bolus tube feedings as the boluses can be timed according to the timing of the diagnostic tests or procedure.
Propofol emulsion contains approximately 0.1 g of fat (1.1 kcal) for every milliliter. An infusion of propofol may therefore provide a significant caloric load. In patients receiving high dose propofol infusions, the enteral feeds need to be adjusted to take into account the added caloric load. A low-fat enteral formulation, such as Vivonex or Vital High Protein may be used.
How Many Calories and How Much Protein to Give?
While no nutrition is bad (starvation) the unresolved question is how much is enough? Adequate nutrition is essential for the critically ill patient in order to ameliorate uncontrolled catabolism, maintain the integrity of the gastrointestinal tract, maintain a competent immune system, and ultimately improve patient outcome. Nutrition support attenuates the metabolic response to stress, limits oxidative cellular injury and favorably modulates the immune response [18–22]. However, the most important goal of nutrition is to promote anabolism and limit protein breakdown; as will be demonstrated further in this chapter none of the current methods of nutritional support have been demonstrated to promote protein synthesis. This would suggest that the amount of protein and calories delivered does not matter; however as reviewed further in this chapter, this is because the standard approach to nutritional support (continuous enteral feeding) does not support anabolism; wrong formula and wrong method of delivery!!
Aberda and colleagues conducted a large observational study to determine the association between the provision of energy and protein and patient outcomes [23]. Data were collected on 2,772 mechanically ventilated patients who received an average of 1,034 kcal/day and 47 g protein/day. An increase of 1,000 cal per day was associated with reduced mortality [OR for 60-day mortality 0.76; CI, 0.61–0.95, p = 0.014] and an increased number of ventilator free days. The benefit of increased calories was only seen in patients with a BMI < 25 and > 35 (moderate obesity may be protective against critical illness). Similar results were observed when comparing increasing protein intake and its effect on mortality. Furthermore a number of studies have demonstrated that the energy deficit accumulated by underfeeding patients during their ICU is an important factor in increasing the risk of adverse outcomes [24–26]. However, it is important to recognize that these are all observational studies that are likely confounded by severity of illness; less sick patients tolerate enteral nutrition better, are more adequately fed and have better outcomes. Four RCT’s support this contention; the first two studies randomized patients to trophic feeds or full nutrition, the third randomized patients to permissive underfeeding or full nutrition while the fourth randomized ICU’s to a “PepUP protocol” where additional nutrition was given to reach caloric targets (in the intervention ICU’s) compared to control ICU’s where patient received usual care. The EDEN study randomized patients (n = 1,000) with acute lung injury to receive either trophic feeding at 20 kcal/h (which is about 7 cal/kg/day) or full feeding at 25–30 kcal/kg/day for the first 6 days [8]. After day 6, all patients who were still receiving mechanical ventilation received the full feeding protocol. There was no difference in the number of ventilator free days (primary outcome), 60 day mortality and other secondary end-points between groups. Follow-up of these patients showed no difference in physical function, psychological and cognitive function as well as quality of life at 12 months [9, 27]. The results of the EDEN trial are similar to a smaller study (n = 200) conducted by Rice and colleagues with a similar study design and patient population to the EDEN trial [28]. Arabi and colleagues performed a two by two factorial design study in which 240 ICU patients were randomized to permissive underfeeding or target feeding (caloric goal: 60–70 % compared with 90–100 % of calculated requirement, respectively) and either intensive or conventional glycemic control [10]. Hospital mortality was lower in the permissive underfeeding group than in the target group (30.0 % compared with 42.5 %; RR 0.71; 95 % CI: 0.50, 0.99; P = 0.04). Heyland et al. randomized 18 ICU’s in the USA and Canada (1,059 patients mechanically ventilated >72 h) to a novel feeding protocol (PEP-uP protocol) which targeted a daily caloric goal or usual care [29]. In the intervention ICU’s enteral feeds started at a higher initial rate and they operationally shifted from an hourly rate to target a 24-h volume goal. This intervention allowed the nurses to increase the volume of feeds delivered if there was an interruption for non-gastrointestinal reasons. While patients in the intervention ICU’s received more calories and protein there was no difference in any outcome variable between the intervention and control ICU’s. These data suggest that achieving target caloric goals (by conventional feeding) does not improve patient outcomes.
The current SCCM/ASPEN guidelines recommend providing 1.2–2.0 g/kg/day of protein [30], with some authorities recommending up to 2.5 g/kg/day to “promote muscle anabolism” [31]. However, increased provision of protein by conventional feeding has not been demonstrated to limit muscle wasting, loss of lean body mass or improve clinical outcomes [29, 32, 33]. Paradoxically, the increased provision of protein either enterally or parentally has been demonstrated to accelerate loss of muscle mass (see below). In a post hoc analysis of the EPaNIC study, Casear et al. demonstrated a strong association between increasing cumulative protein intake with a lower likelihood of an earlier alive-discharge from the ICU [34]. These data suggest that the current guidelines for the provision of protein in critically ill patients are likely to be harmful.
Muscle Wasting in Critical Illness
Survivors of critical illness suffer from marked muscle wasting which may take years (if ever) to recover. The loss of muscle mass is associated with muscle weakness, prolonged mechanical ventilatory support, fatigue and delayed recovery. This disorder is known as Critical Illness Myopathy (CIM). CIM is characterized by a diffuse non-necrotizing myopathy accompanied by fiber atrophy (particularly type-II fast twitch fibers), fatty degeneration of muscle fibers and fibrosis [35]. In the critically ill patient multiple factors are likely to play a role in inducing muscle atrophy including muscle inactivity, inflammation, cellular energy stress, corticosteroids, hyperglycemia, neuromuscular blocking agents and inadequate provision of amino acids (low quality and continuous rather than bolus feeding) [35]. Clinically, patients with CIM may demonstrate weakness, failure to wean or paresis [35]. Creatinine phosphokinase (CPK) levels are relatively normal, consistent with a myopathy and not a myositis. Herridge et al. evaluated 109 survivors of ARDS for up to 5 years after discharge from the ICU. At the time of discharge from the ICU patient’s had lost on average 18 % of their base-line body weight [36]. Seventy-one percent of patients returned to their base-line weight by 1 year. However, it is likely that most of the mass gained was fat mass rather than repletion of the loss of muscle mass [37]. All patients reported poor function and attributed this to the loss of muscle bulk, proximal weakness, and fatigue. At 1 year the distance walked in 6 min was 66 % of predicted which increased to 76 % of predicted at 5 years [38]. The mean score for the physical component of the SF-36 Heath Survey was 25 at 1 year and 41 at 5 years (mean norm score matched for age and sex is 50). At 1 year 48 % of patients had returned to work which increased to 77 % at 5 years. Similarly, Needham and colleagues evaluated the physical performance of patients with ARDS who were enrolled in the EDEN study at 12 months following hospital discharge [9, 27]. At 12 months, the mean 6-min walk test distance was 66 % of predicted while the SF-36 physical health summary score was 39 (mean norm score of 50).
In health, muscle synthesis is stimulated in the post-prandial state while protein breakdown occurs between meals. In healthy individuals muscle mass is maintained through balanced protein breakdown and synthesis. Distinct metabolic pathways are involved in the synthesis and degradation of muscle (see Fig. 32.1). In critical illness, loss of muscle mass results from an imbalance between muscle proteolysis and protein synthesis, with proteolysis overwhelming an inadequate synthetic response. Proteolysis is achieved by several cellular signaling networks, but the predominant proteolytic pathway activated in models of muscle atrophy is the ubiquitin–proteasome system. Two muscle specific E3-ligases belonging to the ubiquitin-proteasome complex, muscle RING-finger 1 (MuRF1) and muscle atrophy F-box (MAFbx) have been identified as key regulators of proteasome-mediated protein breakdown [35, 39–41]. Forkhead box O (FOXO) is a transcriptional factor that plays a major role in muscle wasting primarily by increasing expression of MuRF-1 and MAFbx (atrogen-1) [42, 43]. FOXO dependent gene activation can be regulated by increased overall expression and by posttranslational modification of the transcription factor. Phosphorylation and dephosphorylation of FOXO result in inactivation and activation respectively of the transcription factor. FOXO-1 is activated (dephosphorylated) by inflammation and sepsis. Myostatin, a transforming growth factor-β (TGF-β) protein secreted by muscle induces muscle wasting by directly inhibiting phosphorylation of Akt and thereby increasing the activity of FOXO1 transcription factors and the downstream targets MurF1 and MAFbx. Insulin and insulin growth factor 1 (IGF1) promote net protein synsthesis. In skeletal muscle, the binding of IGF-1 or insulin activates the phosphoinositol-3 kinase/ protein kinase B (PI3K/AKT) pathway inducing muscle hypertrophy by stimulating translation via mTOR kinases. In addition IGF-1 suppresses MuRF1 transcription in part via the phosphatidyl-inositol 3 kinase/AKT pathway. Akt phosphorylates FOXO1 which is then sequestered in the cytoplasm preventing transcription of FOXO target genes [44]. In addition to activation by insulin and AKT, mTOR is controlled by the supply of amino acids and glucose [45].
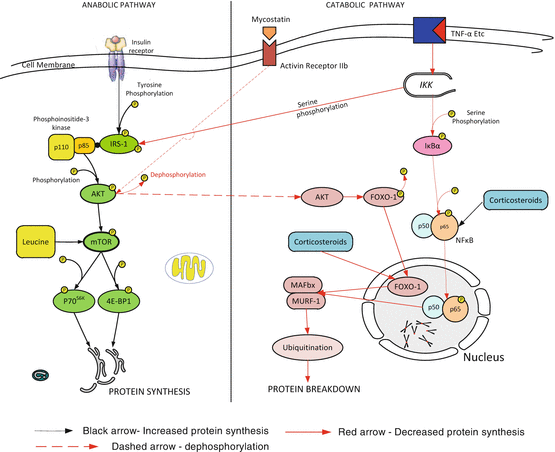

Fig. 32.1
A simplified overview of the anabolic and catabolic pathways in skeletal muscle. Black arrow—Increased protein synthesis. Red arrow—Decreased protein synthesis. Dashed arrow—dephosphorylation. AKT protein kinase b, FOXO–1 forkhead box class O-1, IRS–1 insulin receptor substrate-1, MAFBx muscle atrophy f-box-1, MURF-1 muscle ring finger protein 1, NF κB nuclear factor κB, IKK inhibitor of nuclear factor κB kinase, IκB inhibitor of nuclear factor κB, 4E-BP1 eukaryotic initiation factor (eIF) 4E binding protein 1, P70 S6K 70-kDa ribosomal protein S6 kinase, mTOR mammalian target of rapamycin, TNF-α tumour necrosis factor-α
Puthucheary and colleagues demonstrated a 17 % reduction in the rectus femoris cross sectional area in critically ill patients after 10 days of mechanical ventilation [46]. Loss of muscle mass was greatest in those with multisystem failure and increased with increasing length of stay. Protein synthesis was depressed on day 1 compared to rates in fasted control subjects but increased by day 7. In this study the pattern of intracellular signalling demonstrated increased breakdown and decreased synthesis. Wollersheim and colleagues investigated the dynamics of myosin degradation in patients requiring mechanical ventilation for at least 15 days [35]. These authors demonstrated decreased gene expression of the myosin heavy chain isoforms with significantly increased expression of MuRF-1, MAFbx and FOXO-1 mRNA. Constantin et al. reported similar findings; in addition these authors reported widespread dephosphorylation (inactivation) of the proteins regulating translation initiation factor activation and protein synthesis (AKt1, mTOR, 4E-BP1) and increased expression of myostatin [47]. In an animal model Smith et al. demonstrated that sepsis upregulated FOXO1 expression, by a glucocorticoid-dependent mechanism [42]. Levine and colleagues demonstrated similar findings in the diaphragms of organ donors [41].
While one could argue that providing carbohydrate and fat intravenously (TPN) is unphysiologic and associated with adverse outcomes one could speculate that the provision of protein (amino acids) would promote muscle synthesis. EPaNIC was a large randomized controlled study that compared the early initiation of TPN (early TPN group) when enteral nutrition was insufficient with delayed parenteral nutrition which was initiated after 7 days, allowing a pronounced nutritional deficit to occur in the late TPN group [4]. Casaer et al. evaluated the impact of early TPN on muscle and adipose tissue compartments in a subgroup of patients enrolled in the EPaNIC study [32]. Over the first week there was a 6.9 % loss of femoral muscle volume in both groups. However, as opposed to near starvation in the late TPN group, early TPN reduced the quality of muscle tissue due to increased water and lipid content. In the study by Puthucheary et al. (referenced above) a higher protein delivery during the first week of critical illness was associated with greater muscle wasting [46]. These studies together with those previously reviewed, suggest that the traditional method of feeding patients whether enterally or parenterally does not preserve muscle mass.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





