Introduction
The importance of providing adequate nutrition as an adjunct to medical care was identified as early as the era of Hippocrates. Today, it is known that malnutrition is associated with increased infection rates, longer hospital length of stay, and increased hospital costs. Not surprisingly, malnutrition is also associated with increased mortality. For example, Correia and Waitzberg demonstrated that malnutrition is an independent predictor of mortality, morbidity and hospital expense after controlling for patient age and disease severity. Hence, it is imperative that the nutritional statuses of all patients be assessed throughout their hospitalizations in order to develop appropriate nutritional plans. This could potentially improve outcomes.
Unfortunately, the prevalence of malnutrition in the hospitalized patient was largely ignored until 1974 when Butterworth published his landmark paper entitled “The Skeleton in the Hospital Closet.” Shockingly 30 to 50% of inpatients are malnourished upon admission, and they tend not to improve nutritionally, and frequently worsen, while hospitalized.
Nutrition Evaluation and Screening
Every patient should have a nutrition assessment when admitted to the hospital. This is usually done by a dietitian or a nurse making use of a nutrition screening questionnaire. The assessment process includes a combination of anthropometric measurements, history and physical examination, and laboratory analyses. Utilization of any one parameter is unlikely to yield a reliably accurate determination of nutritional status all of the time. However, when all of these parameters are considered in conjunction, one can generally get a good idea of a patient’s nutritional status.
Every patient should have a nutrition assessment when admitted to the hospital.
Pitfalls to relying on body weight as an indicator of nutritional status include
|
Classically, nutritional status is assessed with anthropometric measurements. These area set of noninvasive, quantitative techniques for determining an individual’s body fat composition by measuring specific dimensions of the body. This includes such parameters as height and weight, triceps skin-fold thickness, mid–upper arm circumference, and bodily circumference at the waist, hip, and chest. If such measurements fall below standard norms then one is considered malnourished. For example, healthcare professionals can compare a patient’s weight to the “ideal” body weight (IBW) found in tables from various sources, such as the 1952 Metropolitan Life tables of IBW. Alternatively, one can use the Hamwi “rule of thumb,” which was developed from the Metropolitan table. This rule states that the IBW for a woman is 100 pounds for the first 5 feet plus an additional 5 pounds for every inch over 5 feet. The IBW for a man is 106 pounds for the first 5 feet plus an additional 6 pounds for every inch over 5 feet.
Body mass index (BMI, weight in kilograms divided by height in meters squared) can also be used as a metric of IBW. A BMI less than 18 kg/m2 is considered underweight, and overweight is a BMI greater than 25 kg/m2. BMI is beginning to replace the other traditional indices of determining IBW because it is independent of gender and body frame size.
Patients who are deemed underweight by any of the foregoing assessments are at risk for developing nutritionally related complications, especially in the presence of acute illness or weight loss. However, one cannot assume that those patients who have a normal weight or who are overweight are well nourished. Acutely ill patients, who have received aggressive resuscitation, can accumulate several kilograms of fluid, which masks their true dry weight and therefore nutritional status. Thus the underweight weight patient may have a “normal” weight under such conditions. Similarly, patients with liver dysfunction and ascites, or renal dysfunction may have their true dry weight masked by excessive fluid retention. Therefore, the physical exam should include assessment for edema and ascites, and comparisons of weight over time should be based on best estimates of dry weight. Finally, even patients whose dry weight is in the obese range can be malnourished. Overweight and obese individuals may have unintentionally lost significant weight over a short period of time due to illness, during which time protein degradation and loss of lean body mass have occurred.
To avoid these pitfalls of determining nutritional status based on admission weight alone, the practitioner should first determine the presence or absence of unintentional weight loss in all patients. Unintentional weight loss has consistently been shown to be a reliable measure of malnutrition, especially in older adults. A loss of greater than 5% of one’s usual body weight (UBW) in 1 month or 10% of UBW over 3 months is considered clinically significant even if one is initially obese. The practitioner should be wary of self-reported height and weight as they are notoriously inaccurate. Hence it is crucial to actually measure height and weight in all patients on admission if possible. Additional physical assessment should include evaluation of skin, hair, mouth, and nails for signs of nutrient deficiencies. Temporal or interosseous muscle wasting and clavicular prominence are classic signs of loss of lean body mass. Dry skin or rash, dry hair or hair loss, poor skin turgor, night blindness, glossitis, and muscle weakness may also point to micro or macronutrient deficiencies. The physician should also determine whether there has been a change in functional status, change in oral intake, or alteration of gastrointestinal (GI) function. These physical and history assessments combined with anthropometric measures assist the healthcare professional with identifying malnourishment.
|
Measurement of serum proteins is also frequently used in the nutrition screening process. Most commonly used are albumin and prealbumin (or transthyretin), but transferrin and retinol-binding protein (RBP) may also be monitored.
Albumin is a well-known marker of nutrition status. It is most useful as an indicator of chronic malnourishment, particularly in the outpatient setting. It is also negatively correlated with morbidity and mortality for inpatients. These positive features make albumin a favorite for nutritional screening. However, albumin is a poor marker of the return of nutritional status to normal in malnourished patients because of its 21-day half-life. One would not expect normalization of serum albumin for at least 14–21 days of adequate protein intake in the hospitalized, malnourished patient, and this is only after the primary pathological process has resolved.
Albumin is a poor marker of the return of nutrition status to normal in malnourished patients because of its 21-day half-life.
Prealbumin is a better marker of nutrition progress in the hospitalized patient due to its 3-day half-life.
|
In contrast to albumin, prealbumin has a 3-day half-life and is a better marker of nutrition progress in the hospitalized patient. Prealbumin has also been shown to be a useful nutrition screening tool, which correlates well with dietitian assessments of nutritional status. Thus, when available, many institutions prefer prealbumin as the laboratory parameter of choice for nutritional screening and monitoring. Transferrin and RBP have been shown to have similar responses to adequate protein intake as prealbumin, and may be substituted in facilities that do not have laboratory assays for prealbumin readily available.
Of note, all of the “nutrition” proteins are negative acute phase reactants. The liver decreases their production during acute illness or following surgery. Hence, a low prealbumin during such periods may be more reflective of an inflammatory condition alone rather than poor nutritional status. In order to optimize interpretation of prealbumin, one should analyze this lab value in concert with C-reactive protein (CRP). CRP, in contrast to prealbumin, is a positive acute phase reactant, which increases during inflammatory states. When CRP is high, prealbumin is usually low. Determining the nutritional significance of a single low prealbumin level in the setting of a high CRP level is unreliable. However, following the trend of several prealbumin and CRP measurements over time can provide useful information. In a patient who is recovering from illness and receiving adequate protein, one should see a reciprocal rise in prealbumin over time and a drop in CRP. If the prealbumin remains depressed despite a return of CRP to normal, it suggests that the patient has inadequate nutrient intake.
Hyper- or hypovolemia, steroid administration, alcoholism, liver and renal failure can also alter circulating nutrition protein levels independent of nutritional status. Measurement of these protein levels on the day of admission and prior to any medical interventions may provide the most relevant information regarding nutritional standing, while measurements several days into a hospital admission are less reliable.
In short, there are many nonnutritional factors that may alter the level of circulating nutritional proteins used to monitor nutritional status. Hence, although it is important to monitor such levels, physicians must use caution when interpreting these markers.
The Nutrition Prescription
Following nutrition assessment, a nutrition prescription is made. No formal nutrition plan is necessary for patients who are well nourished and are not at risk for malnourishment on admission. However, these patients should be reassessed every 3 to 5 days for the development of such risks. All patients who are malnourished on admission, or who are at risk of developing malnutrition, should have a formal nutrition plan developed. This begins with determination of nutrient requirements.
While it is obvious that underfeeding the hospitalized patient is undesirable, it is less well appreciated that overfeeding calories is detrimental as well. Excess carbohydrate administration can result in hyperglycemia and excess carbon dioxide production, which is of particular concern in patients with lung disease who may retain CO2 and have difficulty weaning from the ventilator. Long-term overfeeding may eventually lead to the development of hepatic steatosis. Overfeeding protein can increase ureagenesis and cause renal distress. Overfeeding fat can be immunosuppressive. Hence the goal is to determine nutrient needs precisely, avoid both under- and overfeeding of the hospitalized patient, and deliver macronutrients in the appropriate proportions.
The “gold standard” for determination of caloric requirements is indirect calorimetry, or a “metabolic cart” study. The metabolic cart measures oxygen consumption and carbon dioxide production and then calculates resting energy expenditure (REE) through utilization of the Weir equation. Indirect calorimetry provides a result, which already includes the additional caloric expenditure related to disease stress, but not the caloric expenditure related to activity. Hence the results of the metabolic cart study are increased by an activity factor to calculate the final daily caloric expenditure. Typically, activity factors range from 0 to 5% in intubated patients to as high as 30% in ambulatory patients.
Metabolic cart testing remains the only reliable way to determine caloric requirements for patients with a very low or high BMI, for those with amputations, and for those who have severe illness or injury such as multiple traumas or burns. Use of predictive equations to estimate caloric requirements in such patients can lead to highly inaccurate results. Unfortunately, metabolic cart studies require trained personnel and expensive equipment, so these studies are not available in all facilities.
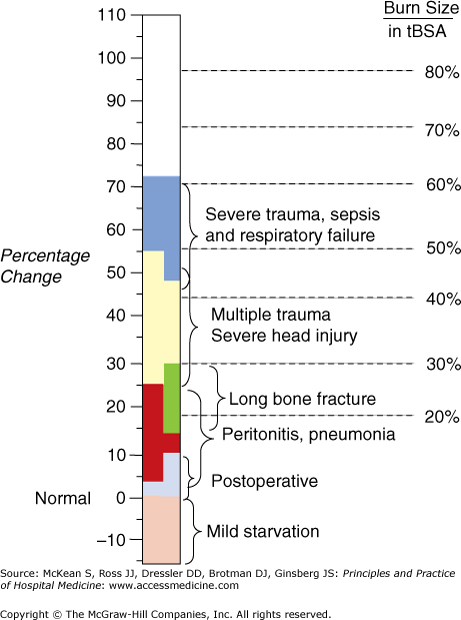
The use of predictive equations is the most common method for determining caloric requirements. There have been many equations developed over the past century. The Harris-Benedict equation (HBE), developed in 1919, is based on studies of healthy volunteers and is the oldest and most widely used equation for determining basal metabolic rate (BMR), the basis for calculating daily caloric requirements. A recent meta-analysis, however, suggests that the Mifflin–St. Jeor equation may be more accurate than the HBE for determining caloric needs when the two equations are compared to indirect calorimetry. The HBE and Mifflin–St. Jeor equations do not factor in the increased caloric expenditure related to disease. This is in contrast to a metabolic cart study. Hence, once the BMR is determined via these predictive equations, an estimate of the additional caloric expenditure from activity and metabolic stress is factored in to determine the overall daily caloric requirement. Stress factors can range from 10% in routine patients to 100% in severe burn patients (Figure 54-1).
Generally speaking, the actual body weight is used for caloric assessment. However, in patients who are greater than 120% of their ideal weight, the use of ideal weight is recommended. Some nutrition professionals will use an “adjusted weight” for obesity, although no routine standardized adjustment has been published (Figure 54-2). In addition to caloric prescription, providers should determine their patients’ protein goals. Generally, hospital patients require between 1.2 and 1.5 g/kg of protein per day, with burn patients requiring up to 2.0g/kg. Provision of protein over 2.0 g/kg/day of actual body weight is not beneficial and may place undue stress of the kidneys.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree