20 Dan Miulli At the moment of neurologic injury, only half of the damage that is going to happen occurs. After admission to the medical system, when the remainder of the harm can occur, is when the biggest impact in care can be made. Guidelines for the Management of Severe Head Injury1 provides information for the optimal care of patients with severe head injuries, from appropriate oxygenation, blood pressure, and intracranial pressure (ICP) to nutrition. Much of the information given in this chapter is drawn from this source. Adequate nutrition is critical to maintaining internal systems, meeting the metabolic demands of increased activity in illness, and helping in the repair and healing of wounds. Considering all the secondary insults occurring in the patient while in the NICU, nutrition may play an even larger role than once imagined, especially if it is considered not as an adjunct but as a necessary therapy.2–4 Nutritional management is a high priority in the NICU setting. Injury to the central nervous system (CNS) can stem from trauma, metabolic disorders, stroke, ischemia, neoplasm, and neuromuscular dysfunction, all of which in the acute stage can significantly increase metabolic needs. Yet nutritional support has not been considered a primary treatment modality; subsequently, the incidence of malnutrition in NICU patients may be as high as 50%.5 Thus, in all admissions to the NICU, nutrition must be addressed in the management of CNS-injured patients. Contrary to what was once thought, after neurologic injury, there is a profound systemic hypermetabolic reaction that results in the rapid depletion of whole-body energy stores. If not attended to, catabolic injury cascade results in the degradation of the integrity of gastrointestinal (GI) mucosa, loss of muscle mass, reductions in systemic protein stores and synthesis, and compromise of both humoral and cellular immune competence. Nutrition can help the patient with neurologic injury by improving ventilator function, speeding wound healing, and fighting secondary infections. The results of not adequately feeding patients are the following: Undernutrition is dangerous; as outlined in the preceding list, it has been shown to decrease the absolute number of circulating T cells, promote cutaneous anergy, and depress the production of new antibodies in response to a novel antigen. The depression of the immune response caused by undernutrition can significantly increase the probability and virulence of infectious complications in surgical patients. Energy and fat stores allow the body to function for a long time without devastating effects. The earliest studies from Studley6 show that a 30% preoperative weight loss led to a 10-fold increased morbidity and mortality following gastric surgery. By extrapolation, using fuzzy logic, there is the assumption that a 15% weight loss in the bedfast patient is of little consequence, bearing in mind a 30% weight loss is potentially very deleterious. Nutritional problems are usually only discussed after visual signs of weight loss, which is the reason nutrition during the first several days to as long as 2 weeks following the injury has not been studied. Three randomized class I studies have evaluated the relationship between caloric intake and patient outcomes.7–10 Rapp et al9 showed that the consequence of severe undernutrition for patients for a 2 week period after injury was an increased mortality rate when compared with those individuals who had full caloric replacement by 7 days. In Young et al’s study,10 there was no difference in those patients who were fed parenterally at 3 days when compared with those fed enterally at 9 days. The preceding chapter discusses weight and weight loss. It is necessary to know how to determine weight in order to determine if there is weight loss. The ideal body weights for people with a medium frame are the following: When protein calorie malnutrition occurs, there is a depletion of skeletal and visceral muscle in a 30:1 ratio. The measured level of the serum protein albumin can approximate visceral muscle stores. Mortality risk increases by ~37% for each gram deficit of serum albumin. Decreased albumin is also responsible for altering colonic resorption of water and salt, manifested by edema and ascites, causes gastric stasis, prolonged small bowel retention, delayed wound healing, and increased wound infection. When a patient has not been fed or when a patient has been hypermetabolic because of neurologic injury, a nutritional blood test panel should be obtained that includes albumin, prealbumin, transferrin, total iron-binding capacity, and serum creatinine. To further help with nutritional assessment, there should be strict monitoring of total input and output as well as daily weights (Table 20–1). Nutrition must not be thought of as preventing large stores of excess fat from being lost but as a form of therapy. At the same time, nutrition must not be haphazardly given, for it has side effects just as any drug does. The nutritional formula must be beneficial and aid in treatment of the neurologically injured patient.
Nutrition
 Need for Nutrition
Need for Nutrition
 Undernutrition
Undernutrition
| Test for nutrition | Normal values | Meaning of low value |
| Serum albumin | 3.5–5.5 g/dL | <3.5 g/dL: compromised protein status, fluid overload, stress |
| Prealbumin (transthyretin) | 14.0–42 mg/dL after 60 years/o 20% lower | <14.0 mg/dL: protein depletion |
| Transferrin | 215–415 mg/dL | <200 mg/dL: malnutrition |
| Total iron-binding capacity | 270–400 μg/dL | <270 μg/dL: compromised protein status |
| Serum creatinine | 0.6–1.6 mg/dL | < 0.6 mg/dL: muscle wasting due to calorie deficiency |
| Total input and output | Euvolemic to slightly hypervolemic | Dehydration and resultant hypotension |
| Daily weights | Loss of fluid or undernutrition |
 Hypermetabolism following CNS Injury
Hypermetabolism following CNS Injury
Patients have an inflated hypermetabolic response to CNS injury. Hadley et al7,8 demonstrated a mean resting energy expenditure 46% above the normal predicted basal metabolic rate in a study of 45 head-injured patients. Most other investigators who measured metabolic expenditure in rested comatose patients also demonstrated ~120 to 250% metabolic rate of the predicted normal basal metabolic rate. During these times, to meet the needs of the increased energy production, there is a marked increase in gluconeogenesis and hepatic protein synthesis and an increased utilization of proteins, carbohydrates, and fats. The increased utilization of fats, carbohydrates, and proteins not only makes supplying nutrition difficult, but it also accelerates the development of malnutrition in patients who are not being fed. In the CNS-injured patient, there is a change in the ratio of nutrients required, an increased demand for protein and lipid calories, and a relative decrease in carbohydrate needs. These requirements must be taken into account when designing a nutritional formula for the CNS-injured patient.
The basic requirements in neurologic injury are
- Increased metabolism
- Increased proteins
- Increased lipids
- Decreased carbohydrates
The adaptive response to CNS injury is an increase in catecholamines, cortisol, and glucagons, with a subsequent increase in metabolism and hyperglycemia.8 Both gluconeogenesis and lipolysis continue. Even with hyperglycemia, 75 to 90% of energy is still supplied by fat oxidation. In moderation, fat oxidation is not a major problem; however, when there is decreased blood return and continued fat oxidation, there is free radical production and further neurologic damage. Although protein is required for the repair of the injured brain, the changes in catecholamines, cortisol, and glucagon lead to proteolytic metabolism. Catecholamines are released and, in turn, stimulate the release of adrenocorticotropic hormone, growth hormones, glucagons, and insulin. Catabolic hormones, such as glucagons, cortisol, and catecholamines, cause utilization of alternate energy sources when there are no further nutrients supplied. As the injury continues, stress increases and proteolysis persists, making it impossible to achieve a positive nitrogen balance in the early period after CNS injury. Even if CNS-injured patients are paralyzed or put into barbiturate comas, they still require 100 to 120% of the resting energy expenditure.11 The increase in resting energy suggests that a major part of the elevated metabolic expenditure is due to muscle tone.12
The normal responses to CNS injury are
- Increased catecholamines
- Increased cortisol
- Increased glucagons
- Proteolysis
- Gluconeogenesis
- Lipolysis
- Increased adrenocorticotropic hormone, growth hormones, and insulin
- Proteolysis
Table 20−2 GI Losses following CNS Injury2–5
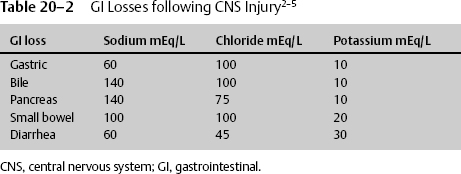
In addition to the loss of protein stores throughout the body, injury results in other changes, such as electrolytes being lost from GI tract secretions. Just as protein stores must be replaced, electrolytic losses must be replaced in nutritional therapy (Table 20–2).2
 How Much Nutrition Is Needed?
How Much Nutrition Is Needed?
The ballpark figures for determining normal caloric requirements is 30 kcal per kg per day for GCS >13 to 15, 35 kcal per kg per day for GCS = 8 to 12, and 40 to 45 kcal per kg per day for GCS = 3 to 7. Indirect calorimetry performed with the aid of a portable metabolic cart once a day for the first several days is the most accurate way to determine the patient’s resting energy expenditure and nutrition requirements. This method is unnecessary, however, because the general energy requirements can also be determined using the Harris-Benedict equation (Table 20–3), although that formula has a systematic error rate of 5 to 15% overestimation.
Clifton et al13 simplified the adjustment factors, correcting the Harris-Benedict overestimation. They showed that the required percentage of resting energy expenditure of total calories calculated from part I of the Harris-Benedict formula can be estimated accurately using GCS, heart rate (HR), and days since injury. The percentage of required energy expenditure as determined using the Clifton equation is
152 − [14 × GCS] + 0.4 × HR + 7 × the day since injury.
| Men |
| Part I: [66.47+(13.75 × weight in kilograms) + (5.0 × height in centimeters) − (6.76 × age in years)] = basal calories |
| Part II: Multiply basal calories by adjusted activity factor, then by injury factor = the required energy expenditure in total calories per day |
| Women |
| Part I: [655.10 + (9.65 × weight in kilograms) + (1.85 × height in centimeters) − (4.68 × age in years)] = basal calories |
| Part II: Multiply basal calories by adjusted activity factor, then by injury factor = the required energy expenditure in total calories per day |
Source: From Page CP, Hardin TC. Nutritional Assessment and Support: A Primer. Baltimore, MD: Williams & Wilkins; 1989.
Activity factors: confined to bed: 1.2; out of bed: 1.3.
Injury factors: minor surgery: 1.1; major surgery: 1.2; infection, mild: 1.2; infection, moderate: 1.4; infection, severe: 1.8; trauma, skeletal: 1.35; head injury, with steroids (increase metabolism): 1.6; head injury, blunt: 1.35; burns, 40% 1.5; burns, 100%: 1.95.2–4
In the first 2 weeks after injury, energy expenditure rises regardless of neurologic course, but its extent beyond this is unknown. The hypermetabolic response is also prolonged in patients who have other major organ system trauma. This hypermetabolism can be exaggerated for up to 1 year in severe head-injured patients; however, this should not be thought of as the norm.
In the neurologically injured patient, the resting energy requirements will be supplied by muscle breakdown or other body stores of proteins, fats, and carbohydrates (Table 20–4).
Initially in the neurologically injured patient, often the hypermetabolic state is combined with undernutrition, compromising protein synthesis and resulting in lean muscle mass catabolism for essential amino acids. As the hypermetabolism/hypercatabolic process continues, nitrogen is lost through the urine and feces. There is essentially no nitrogen gained through normal levels of nutrition, but a great deal of nitrogen is lost, leading to a negative nitrogen balance. For each gram of nitrogen in the urine and feces, 6.25 g of protein has been catabolized. Nonfed patients with severe brain injury continue to lose 14 to 25 g of nitrogen per day. The maximum excretion occurs during the second week. The average nitrogen loss of a head injury patient is 0.2 g of nitrogen per kilogram per day, which will result in a 10% loss of lean mass in 7 days.8 This is about double or triple the loss in the normal person. Unlike the Studley6 article, which showed that a 30% preoperative weight loss led to increased morbidity and mortality following gastric surgery, there are no studies that examine nitrogen loss or nitrogen replacement with outcome; therefore, nitrogen balance may not be a major issue. Nonetheless, a study by Clifton et al14 examined two matched groups of comatose head injury patients and determined that the level of nitrogen intake should be 20% of the core composition of a 50 kilocal/kg/day feeding protocol. For the hypermetabolic patient, 20% is the maximal protein content of most enteral feedings; it is also the maximal amino acid content of most parenteral formulations.
| Substrate Providing Calories | Energy (kcal/g) |
| Muscle | 1 |
| Carbohydrate | 4 |
| Protein | 4 |
| Fat | 9 |
- 100 mL/kg for first 10 kg in 24 hours, then
- 50 mL/kg for next 10 kg in 24 hours, then
- 20 mL/kg for remainder weight in kg in 24 hours age < 60 years
- 15 mL/kg for remainder weight in kg in 24 hours age > 60 years
The protein content of the fluid should be 1.0, 1.5, or 2.0 kcal/mL to meet partial nutritional needs and to continue a fluid euvolemic state. There are many physiological circumstances that require increased fluids from the normal amount. Fever increases fluid requirements by 12.5% for each 1°C above normal, sweating 10 to 25%, and hyperventilation 10 to 60%.15
Fluid types must also be used judiciously. The deleterious effects of hypotonic intravenous (IV) solutions on brain injury have been known since 191916; therefore, immediately after neurologic problems have been detected, IV hypotonic fluid such as lactated Ringer’s (Na = 130 mEq/L; 273 mOsm/L), dextrose 5% in water (D5W), and 0.45% sodium chloride (0.45NS) should be avoided. Decreasing IV fluids 1 mEq/L decreases osmotic pressure 38.6 mm Hg16 and can lead to brain edema. It is also known, though, that hypertonic saline solutions lower ICP.17,18
There have been multiple class I investigations of the amount of feeding, the route of feeding, the use of steroids, and nitrogen balance, but none of these studies have looked at patient outcome or complications. Even the evidence of what to feed the CNS-injured patient remains unclear. The formulas must be specifically tailored; even the ingredients once thought to be the foundation of nutritional therapies need to be reexamined. High osmolar total parenteral nutrition (TPN) increases brain edema after cryogenic injury in animals19 because of changes in serum osmolarity. The increased edema does not occur with lower osmolarity TPN. Besides hyperosmolar nutrition causing worse outcome after CNS injury, hyperglycemia is well recognized as worsening outcome after CNS injury and must be avoided.20–23 It has even been suggested that hypoglycemia may be neurocytoprotective.24–26 Hyperglycemia is common in trauma patients, even without supplementation, and is exacerbated by the administration of any TPN, hyper- or iso-osmolar.10 Therefore, TPN should not be given during the first 48 hours after CNS injury, and serum glucose concentrations must be closely monitored and treated vigorously and kept below 110 mg/dL
Nutritional treatments should not deliver less than the minimal daily requirement; As nutritional science matures, nutritional requirements in specific diseased states will be developed.
 The Gut Benefits from Nutrition
The Gut Benefits from Nutrition
Early nutritional support increases CD4 cells, the CD4:CD8 ratio, and the T-lymphocyte responsiveness to concanavalin A (ConA), which assists immune function in patients sustaining CNS injury.27 For nutrition delivery to the body to optimally occur in a physiological manner, the GI tract cells must be functioning. A functioning GI tract is necessary to protect against infection and sepsis from its own bacteria, particularly after CNS injury or surgical stress. If the gut is maintained, there will be no bacterial overgrowth, no proliferation of specific pathogens within the bowel, and no penetration of the pathogens into the bowel wall. Furthermore, with nutrition, the GI tract will remain the principal organ in the regulation of inter-organ amino acid exchange, with glutamine being the most important amino acid available to the GI cells. After CNS injury, the GI tract cells’ uptake of glutamine increases despite decreased oral intake and decreased delivery of glutamine to the intestinal mucosa. Glutamine is the most abundant amino acid in the blood, possibly because it is a precursor for purines and pyrimidines. When there is no added nutrition available, glutamine must come from another source; it originates from the muscle catabolism.
In summary, the primary benefits of maintaining GI mucosa with glutamine nutrition are the following:
- Protects against infection and sepsis from GI bacteria
- Prevents the proliferation of specific pathogens within the bowel
- Prevents penetration of the pathogens into the bowel wall
- Maintains the gut as the principal organ in the regulation of interorgan amino acid exchange
- Necessary for protein production
- Necessary for nitrogen transport and ammonia excretion
- Prevents muscle degradation
- Decreases hypermetabolism
In undernutrition, muscle undergoes proteolysis and releases essential glutamine into the circulation, supplying the basic requirements for GI ente-rocytes and for renal aminogenesis. However, the GI cells quickly use the glutamine from the muscle, thereby decreasing the circulating glutamine level available to other cells of the body for protein production, nitrogen transport, and ammonia excretion. In functioning cells throughout the body, glutamine is catabolized by mitochondrial glutaminase to glutamate and ammonia; once released into the circulation and across the blood–brain barrier, it can destroy brain cells. However, not all glutamine by-products are neurotoxic; alanine, for example, is used by the liver for gluconeogenesis, and ammonia is converted to urea. Circulating glutamine is necessary for the gut to prevent infection; is necessary to cells for protein synthesis; is important for nitrogen and carbon transport, as well as ammonia excretion; is the major substrate for gluconeogenisis and renal angiogenesis; and is a fuel source for rapidly replicating cells. However, too much glutamine can be a problem. Although the by-products of glutamine metabolism can cause neurologic problems, there is no evidence that an exogenous supply to the gut increases neurologic injury when the kidneys and liver are functioning. Therefore, the net overall requirement is for glutamine to be provided during CNS injury as soon as possible at appropriate levels to maintain homeostasis.
 How to Feed CNS-Injured Patients
How to Feed CNS-Injured Patients
Patients can be fed using the stomach (gastric), the small intestines (enteral), and the veins (IV) (parenteral). Each method has advantages and disadvantages. Multiple issues affect each type of feeding. Enteral feeding in the neu-rologically impaired patient is often complicated by medications such as phenobarbital, morphine, and other narcotics that delay gastric emptying. Ott et al28 found that for a mean of 15 days, more than 50% of head-injured patients did not tolerate enteral feedings because of delayed gastric emptying. However, gastric feeding does not have to be the method of choice when utilizing the GI tract. There has been one class I report and one class II report indicating better tolerance of enteral feeding with a jejunal rather than gastric administration. Enteral feeding does have advantages over IV (parenteral) feeding. It is more cost effective, safer, has fewer complications, and is more physiologic than parenteral nutrition.
Indications for enteral nutrition are
- Malnourished patients expected to be unable to eat > 5 to 7 days
- Nourished patients not able to eat for 7 to 9 days
- Patients who are unable to consume adequate amounts of kilocalories to prevent macronutrient and micronutrient deficiencies
Contraindications to enteral nutrition are
- Severe acute pancreatitis
- High output proximal fistula
- Inability to gain access
- Patients expected to eat within 5 to 7 days
- Intractable vomiting and diarrhea
- Aggressive therapy not indicated
- Patients expected to eat within 5 to 7 days
Using the gastrointestinal tract has physiologic superiority, stimulating gut hormones, gastric acid buffering, and buffering supplements into the blood. The advantages of enteral feeding are decreased hyperglycemia, decreased infection and sepsis, and decreased cost. Enteral feeding maintains the gut mucosa integrity, prevents infection, promotes protein synthesis, transports toxins, and decreases hypermetabolism. The only advantage of parenteral nutrition can be seen when enteral feeding cannot be tolerated; immediately preoperatively, with the need for intentional bowel rest, and with bowel dysfunction, the IV route must be used for a short time. IV nutrition can also supplement enteral nutrition when not enough calories and proteins are being provided. Parenteral nutrition aggravates brain edema in laboratory animals and may cause elevated liver or pancreatic enzymes. Lebkowski29 showed that neither parenteral nor enteral nutrition in severely head-injured patients made any difference in outcome. However, the same study also demonstrated that no nutrition made any difference in outcome.
The neurologically injured patient often is treated in the flat bed position, especially if spinal injuries have occurred or vasospasms are present. Most of the time the head of the bed is tilted at 30 to 45 degrees or titrated to the best ICP. When the head of the bed is flat, the patient is at risk of aspiration. If aspirations are suspected, tube feedings should be held until the tube can be placed into the small intestine. Intubation does provide a degree of protection, especially if a subglottic suction endotracheal tube is used. However, a recent study demonstrated ulcers in an animal model; therefore, its use cannot be recommended.30 When there is a concern about possible aspiration, gastric reflux, or a tracheal-esophageal fistula, food coloring can be added for 24 hours to feedings for investigation. In the past, blue food coloring and methylene blue have been added to enteral feedings to detect aspiration. Increased absorption and systemic levels of drugs such as methylene blue can occur when there is increased intestinal permeability. The blue dye then interferes with normal adenosine triphosphate (ATP) production and ultimately inhibits mitochondrial respiration, leading to shock, metabolic acidosis, and cell death.31 Therefore, its use cannot be recommended.
Severely head-injured patients who remain comatose have swallowing difficulties and may be fed either parenterally or enterally. Prolonged use of a naso- or orogastric tube should be avoided because of the risk of mucosa ulceration and sinus disease. If the patient is expected to have feeding difficulties for 1 month or more, a percutaneous endoscopic gastrostomy (PEG) tube should be placed.
Eventually, established feedings should approximate the normal bolus manner of feeding instead of continuous feedings,32,33 although initially, continuous feedings are better tolerated in the critically injured patient.34 If given the choice of gastric or duodenal feeding versus jejunostomy, the more proximal area should be used because a patient receives fewer calories via the more distal jejunostomy tube. This was exemplified by Opeskin and Lee35 in the case of a healthy 16-year-old severely head-injured patient being fed by jejunostomy tube who died because of malnutrition. She had hyperperistalsis that caused the jejunostomy tube to migrate even more distally, resulting in less nutrition being absorbed.
The gut may provide the most efficient and safe route of fluid administration and should be used as the first choice when there are no contraindications. For example, when diabetes insipidus is suspected, an enteric feeding tube can be used to provide the patient water when IV water would never be considered.
Once again, no class I studies have looked at patient outcome, but there are multiple class II to IV studies that provide evidence of outcome. Borzotta et al36 demonstrated that neither enteral nor TPN was effective in changing outcome. In the past, when compared with enteral nutrition, TPN was thought of as a superior means of nutrition because of the misconception that the bowel was atonic. However, Hadley et al7 demonstrated that aggressive enteral feeding could be accomplished early and effectively despite bowel atony. Grahm et al37 added support to using enteral routes when they demonstrated that enterally fed patients had statistically significant lower rates of hospital-acquired infection and sepsis, and shorter stays in the ICU, than patients receiving the same caloric and nitrogen supplements by TPN. The researchers passed a malleable nasojejunal feeding tube directly into the proximal jejunum with the aid of bedside fluoroscopy. They used the nasojejunal feeding tube in spite of a history of blunt abdominal trauma, the use of barbiturates, narcotics, and other agents, and compared the results with TPN. The minor differences that tended to favor enteral nutrition were less hyperglycemia, less of a difference in osmolarity loads causing edema, and lower cost. Enteral nutrition preserved gut mucosa, stimulated complex circulatory hormonal mechanisms that are intrinsic to the human digestive system, and preserved the secretion of the immunoglobulin A (IgA) immune system. Thus the use of enteral nutrition tends toward improved efficacy. Furthermore, enteral feedings are best accomplished if the tube is placed in the jejunum, decreasing chances of aspiration and improving GI tract motility.
Postpyloric enteral feeding requires the following:
- Bowel sounds must be present.
- Bowel movements or flatus is not necessary.
- Nasoduodenal feeding tube must be placed.
- Certain feeding tubes must be confirmed by abdominal x-ray.
- Head of bed must be maintained at a tilt of 30 degrees or greater.
- Check gastric residuals by oral gastric tube; do not aspirate through a small or pliable duodenal tube.
- Bolus flush the feeding tube with at least 30 cc water every 4 hours to prevent clogging.
Small intestinal feedings can reduce the risk of aspiration. In addition to a decrease in infection rates with early enteral feedings, the occurrence of bacterial translocation, or the passage of bacteria across the intestinal wall, is also decreased.
As outlined above, when starting tube feedings, determine first that there are bowel sounds, confirm the placement of certain tubes by abdominal x-ray, place the head of the bed at 30 degrees or more, then determine the total amount of calories or the goal calories and the amount of liquid in milliliters to be given per hour for 24 hours. Begin tube feedings at 10 to 50 cc per hour, full strength. Check for gastric residuals after the first 2 hours, then after every 4 hours for 3 cycles. If the amount of feeding formula residual is greater than 50% of the feedings delivered during the cycle, hold the feedings for 1 hour and recheck every hour until there is less than 50% of the feedings delivered during the last cycle. It is often difficult as well as inaccurate to measure residuals with pliable feeding tubes. If feedings are tolerated, increase the amount 20 to 40 cc every 8 hours until the goal is reached. Make sure that the feeding tube is flushed with 30 cc of water every 4 hours to prevent clogging.
When tube feedings have reached the goal amount and are well tolerated, bolus feeding may begin. Give feedings during normal waking hours every 4 hours. Start with double the hourly rate of the continuous tube feedings. Flush with at least 30 cc water after every bolus, then clamp the feeding tube. Next, follow the same guidelines for checking residuals with continuous feedings. Check for gastric residuals after the first 2 hours, then after every 4 hour cycle. The cycles of bolus feedings may be increased to every 6 hours if fluid requirements dictate. If the amount of residual feeding formula in the gut is greater than 50% of the feedings delivered during the cycle, hold the feedings for 1 hour and recheck every hour until there is less than 50% of the feedings delivered during the last cycle; resume when appropriate.
Discontinue enteral feedings only when oral intake is appropriate. If possible, wean the tube feedings to oral intake; this is often easier with bolus-type delivery. However, some patients do not tolerate oral intake if a nasal feeding tube is in place.
Occasionally, diarrhea occurs with tube feedings. Likely causes are elixir medications containing sorbitol, magnesium-containing antacids, oral antibiotics, phosphorus supplements, cimetidine, metopramide, lactulose, pseudomembranous colitis, and gastrointestinal disorders. When diarrhea starts, measure stool Costridium difficile titers, especially if the stool is very malodorous. Then increase fiber by considering the addition of pectin or psyllium; however, psyllium will clog the feeding tube. When treating diarrhea, do not initially give antidiarrheal agents before the cause is known; otherwise, it will slow down the elimination of the offending agent and prolong the diarrhea. Do not stop the tube feedings for any length of time, and do not change formulas.4
 What Should CNS-Injured Patients Be Fed?
What Should CNS-Injured Patients Be Fed?
Nutrition is a form of therapy. It must be safe, it must prevent secondary injury, and it should improve outcome. Nutrition decreases the risk of infection, maintains the gut mucosa integrity, promotes protein synthesis, transports toxins, prevents muscle degradation, and decreases hypermetabolism. To decrease complications, nutrition should be made slightly acidic because gastric alkalinization significantly increases the risk of nosocomial ammonia in long-term ventilated patients.38 The brain requires ~20% of the body’s resting energy expenditure, and although dependent on glucose, it will utilize ketones under conditions of significant deprivation. Ritter et al39 randomized 20 severely head-injured patients to receive Osmolyte HN (Ross Laboratories, Columbus, OH) or carbohydrate-free tube feeds. The experimental tube feed contained 2060 kcal/L, 129 g/L of protein, and 175 g/L of fat. In these patients, blood glucose and blood lactate were lower, arterial ketones were higher, and daily nitrogen loss was lower. Unfortunately, the investigators did not measure outcome.
To be well rounded, in addition to proteins, enteral nutrition must contain fat and possibly some carbohydrates. Each should be added in moderation. Carbohydrates should not be overly used because hyperglycemia leads to a worse outcome in CNS injury. Large amounts of administered carbohydrates/glucose have several negative effects. First, they will raise the carbon dioxide production because of their relatively high respiratory quotient of 1. The respiratory quotient of protein oxidation is 0.8; fat oxidation, 0.7. The higher respiratory quotient of carbohydrates will result in increased ventilation needs and decreased ability to be weaned. High serum glucose levels will also stimulate lipogenesis and result in hepatic steatosis. Additionally, high glucose increases the resting energy expenditure due to the thermic effect of the large doses of administered carbohydrates.
The use of tight glucose control 80 to 110 mg/dL improves mortality and morbidity. This can be accomplished with a Humulin regular sliding scale IV dosage or much more accurately with an insulin continuous infusion. Continuous infusion of insulin has been shown to provide more steady states, approximating physiological conditions.40–44
Fats
In addition to carbohydrates, fats must be included in feedings. Although Roth et al45 described how a fat emulsion containing 20% intralipid caused life-threatening hemophagocytosis, hypertriglyceridemia, and creaming plasma after a 3 day period of parenteral nutrition in a 21-year-old, fats are important. They provide 8 kcal per gram of fat and normally make up 10% or less of the consumed calories in the modern diet. Diets should contain medium-chain fatty acids because they are well tolerated and do not increase deleterious plasma lipoproteins. Essential fatty acids are required, with each type having advantages and disadvantages. Diets rich in omega-6 polyunsaturated fatty acids, such as those obtained from corn, safflower, and sunflower oils, diminish the immune response to infection, trauma, or tumor growth, as documented by Alexander and Peck.46 Arachidonic acid, a byproduct of fats, breaks down and stimulates oxygen-free radicals and further damage. In contrast, diets abundant in omega-3 polyunsaturated fatty acids, such as those found in coldwater fish oils, stimulate the immune response to infection and trauma and activate the rejection of foreign bodies.
Proteins
Proteins are very important in nutrition and must supply the enormous need for specific amino acids. Saito et al47 demonstrated that arginine is important in neurologic injury. When arginine comprised 2% of the total nonprotein calories in burn-injured animals, it increased survival, improved delayed hypersensitivity, and reduced susceptibility to infection. Arginine is thymotrophic and improves cellular immunity by increasing thymic lymphocytes sensitivity. It also improves wound healing and decreases nitrogen release.8 Amino acids are vital for neurologic healing; for example, inosine has been shown to stimulate axonal growth.48 However, not all amino acids benefit head-injured patients. Theoretically, glutamine and glutamate from the diet can cross a disrupted blood–brain barrier and exacerbate glutamate neurotoxicity immediately after primary injury or during secondary injury, and can lead to the loss of cells in the ischemic penumbra. This has not been proven in human subjects, however. In general, after injury, protein catabo-lism leads to increased nitrogen levels and an increased production of ammonia. However, the levels of nitrogen that lead to increased ammonia and neuronal damage are not known. It is recommended that nitrogen to nonprotein calories in the human diet range from 1:75 to 1:185. Enteral feedings should contain at least 15% of calories as smaller protein by the seventh day after injury. The optimal amount of protein per kilogram of body weight in the neurologically injured patient is unknown. Normal nutrition suggests 1.5 g/kg, studies suggest 2.5 g/kg,49 but it may be as high as 4.5 g/kg. Proteins, electrolytes, and dextrose add osmolarity. Most feedings should have an osmolarity around 300 so that it does not add to edema at lower osmolarities or leak across the damaged blood–brain barrier as higher osmolarity would.
In addition to the main building blocks of carbohydrates, fats, and protein, there should be 1 to 2 calories per milliliter to decrease the fluid load. The nutritional formula should be high in zinc, as this is associated with improved neurologic recovery rate and improved protein levels in patients with severe closed head injury.50 The formula should also be low in iron and high in desferrioxamine. Desferrioxamine prevents the damage associated with free radical generation and reperfusion injury. It inactivates the iron-dependent enzyme ribonucleotide reductase that has been shown to decrease infarct size and improve functional recovery.51 A creatine-enriched formula was shown to help prevent secondary neurologic energy in an animal model.52 Additionally, the nutritional formula must contain vitamins and other elements. Glucose loads should be low because of problems with hyperglycemia; large doses of glucose will suppress lipolysis and prevent mobilization of stored linoleic acid. It may also be prudent to add human growth hormone.53 This has no effect on nitrogen imbalance in highly stressed immobilized patients after severe head injury, but it significantly enhances serum protein concentrations.
 Disease-Specific Formulas
Disease-Specific Formulas
Immune-enhancing formulas with glutamine, arginine, omega-3 fatty acids, and nucleotides have been used recently in critically ill patients. Glutamine and arginine have been shown to decrease infection rates and promote wound healing in the critically ill,54 but they are contraindicated in patients with hepatic and renal failure. Arginine supplementation has immune-enhancing benefits, including increased rate of protein repletion, improved collagen synthesis and wound healing, and enhanced T cell function.
The goal of pulmonary formulas is to reduce carbon dioxide production. These formulas are low in carbohydrates and have a high (50%) fat content. Care must be taken to avoid overfeeding. Total calorie intake has more of an impact on respiratory function than specialized pulmonary formulas.
Hepatic formulas are low in protein to minimize ammonia production. These formulas contain large amounts of the branched chain amino acids valine, leucine, and isoleucine, and low amounts of aromatic amino acids. They assist in treating encephalopathy, especially from ammonia.
Special diabetic formulas are high fiber, low carbohydrate, and high fat to provide nutrition support to patients with hyperglycemia; they may be considered in all neurologically injured patients. However, because of delayed gastric emptying in a head trauma patient, close monitoring of tube feeding tolerance is recommended.
Absorption may be enhanced with the use of elemental formulas if intestinal atrophy or loss of absorptive surface has occurred.
The characteristics of an optimal nutritional formula in the treatment of an acute neurologic injury are
- One to 2 calories per milliliter, depending on the need for euvolemia or hypervolemia
- Slightly acidic
- Small intestine placement
- Osmolarity 300
- High protein containing arginine, inosine, and glutamine
- Fatty acids with medium chain and omega-3 and polyunsaturated fatty acids
- Very low or absent carbohydrates
- Fiber (25–35 g/day)
- High magnesium initially
- High zinc
- Desferrioxamine
- Creatine
- Low potassium initially
- Low calcium (800–1500 mg/day initially)
- Low iron
- High magnesium initially
 Total Parenteral Nutrition
Total Parenteral Nutrition
The advantage of parenteral nutrition can be seen when enteral feeding cannot be tolerated immediately preoperatively, with the need for intentional bowel rest and with proven severe bowel dysfunction. IV nutrition can also supplement enteral nutrition when not enough calories and proteins are being provided.
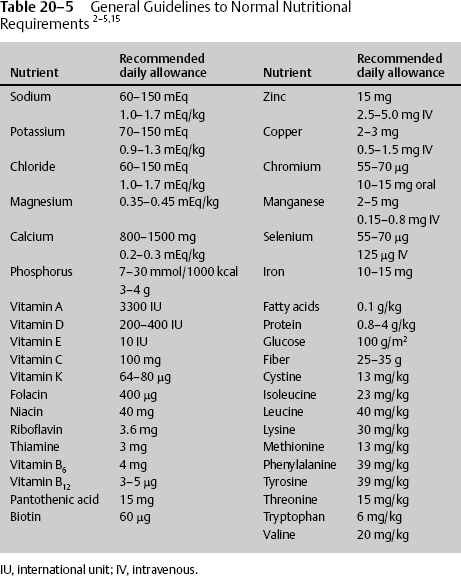
TPN is drug therapy delivered to the IV system without the benefit of the GI buffering system. It must contain the appropriate medications to provide optimal nutrition. Electrolytes, protein, fats, and vitamins are required for health and are provided in most nutritional supplements, but they must be added to IV TPN. The amount that the physician prescribes is dependent upon sex, age, organ losses, and diseased states. The normal requirements thus vary by the same criteria, but general guidelines are given in Table 20–5.
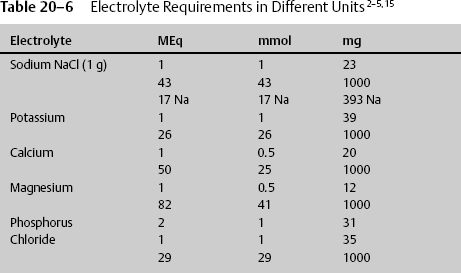
Often electrolytes are prescribed in different units, and the conversion must be known. IV fluids are given in milliequivalents, and dietary supplements are given in milligrams (Table 20–6).
The requirements in the average healthy individual are not the same in the debilitated individual. When providing TPN, the amount of electrolytes must be adjusted for 1000 kcal of TPN (Table 20–7).
Typical central IV TPN must be adjusted for diseased state, determining the beneficial compounds added. The fluid requirements must also be obtained according to the disease state and whether euvolemia or hypervolemia is beneficial. Peripheral administration requires adjustment to prevent peripheral vein injury. As such, the amount of amino acids in peripheral IV feedings should be 35 g, dextrose 70 g, sodium chloride 15 mEq, and heparin 200 units (Table 20–8).
Prior to beginning TPN, make sure that the central venous access line has one port labeled for only TPN. Central line TPN usually contains 70% dextrose, 10% amino acids, and 20% fat emulsion. If peripheral access is chosen, the amount of dextrose is decreased to 20%, amino acids to 8.5%, and fats to 10%. As with the administration of all IV medication, the TPN must be delivered at a constant rate. The TPN delivery system must be maintained sterile. Document the patient’s height, weight, age, sex, heart rate, and GCS; the days since injury; the total caloric goal; and the fluid desired. There are specific TPN formulas commercially available. Start TPN at 50 cc per hour, and increase every 8 hours until the goal can be reached in 24 hours. When discontinuing the TPN, taper the infusion rate by 50% every 30 minutes for 1 hour to prevent hypoglycemia.
TPN is a direct assault on the liver and pancreas and bypasses the stomach, providing electrolytes directly to the bloodstream. TPN causes fatty liver, cholestasis, GI atrophy, and gastric hyperacidity. Therefore, multiple tests should be monitored when delivering TPN (Table 20–9).
| Electrolyte | mEq |
| Sodium | 5–10 |
| Potassium | 20–40 |
| Phosphorus | 10–15 |
| Magnesium | 8 |
| Calcium | 5 |
 Changing Metabolic Conditions
Changing Metabolic Conditions
Formulas must have the ability to change under different metabolic conditions. For example, magnesium is important early in injury treatment, preventing vasospasms and the spread of glutamate neurotoxicity. However, it is not beneficial after 7 to 10 days, when recovery is expected and when cell transmission is needed.
| Ingredients | Average amount (per liter) |
| Carbohydrate dextrose | 250 g |
| Lipids 20% | 250 mL |
| Amino acids | 60 g |
| Sodium given as: | |
| Sodium chloride | 60 mEq |
| Sodium acetate | 60 mEq |
| Sodium phosphate | 60 mEq |
| Potassium given as: | |
| Potassium chloride | 60 mEq |
| Potassium acetate | 60 mEq |
| Potassium phosphate | 60 mEq |
| Magnesium sulphate | 16–24 mEq/day |
| Calcium gluconate | 10–15 mEq/day |
| Multivitamins | 10 mL |
| Vitamin C | 1000 mg |
| Vitamin K | 10 mg/week |
| Trace elements | 4 mL |
| Insulin | At least 15 units/day |
| Zinc, extra | 10 mg |
| Heparin | 1000 units |
| Serum electrolytes, glucose, BUN, creatinine, calcium, magnesium, phosphorus | Daily |
| Albumin, prealbumin, transferrin, total iron-binding capacity, hepatic enzymes, bilirubin, triglycerides, prothrombin time, WBC | Weekly |
BUN, blood urea nitrogen; WBC, white blood (cell) count.
 Additional Concerns for Nutrition
Additional Concerns for Nutrition
Nutritional therapy must be customized for clinical conditions (Table 20–10).
At times, the neurologically injured patient may not have been fed for 1 to 3 days, is inactive, and may often receive narcotics, all of which individually can result in decreased gut motility. Therefore, initial admitting orders for the NICU should include a stool softener such as docusate sodium or the combination capsule casanthranol/docusate sodium. Appropriate bowel movement prevents abdominal distention, pain, anxiety, nausea, and vomiting. Therefore, treat constipation appropriately. Always consider abdominal injury in the multitrauma patient; if not present and if stool softeners do not result in regularly scheduled bowel movements, then bisacodyl suppositories and enemas should be given every other day unless a bowel movement has occurred.
| Syndrome of inappropriate antidiuretic hormone | Decreased fluids |
| Cerebral salt wasting | Increased salt |
| Diabetes insipidus | Decreased salt and increased fluids |
| Ventilators | Increased phosphorus |
| Initial head injury | Increased magnesium |
| Vasospasms | Increased magnesium |
There is no benefit to giving metoclopramide in patients with severe neurologic injury to stimulate gastric motility. It does not prophylactically improve gastric tolerance;55 furthermore, it can cause CNS effects such as neuroleptic malignant syndrome, parkinsonian symptoms, dystonic reactions, and restlessness.
< div class='tao-gold-member'>