The
CDC estimates that in the United States there are 2 million nosocomial infections annually leading to increased mortality with a conservative estimate of an additional $5.7-$6.8 billion (
US dollars) in healthcare costs as a result (
2). Although it may not be possible to eliminate all nosocomial infections, one-third or more could be prevented with implementation of organized infection-control programs. Given the incidence, mortality, morbidity, and cost of nosocomial infections, those which could be prevented but were not may be considered a source of medical error. Despite even the best infection-control program, there will always be the risk of nosocomial infection in the
ICU due to the unique nature of the critically ill or injured patient. However, many adult and pediatric ICUs across the country have achieved zero nosocomial infections for prolonged periods of time.
The risk of nosocomial infection depends on a variety of factors. The location within the hospital plays an important role with the highest rates typically occurring in the
ICU. Even the type of
ICU influences the nosocomial infection rate with different rates seen in medical, surgical, trauma, burn, neurological/neurosurgical, and cardiac ICUs. The
PICU is unique in that it typically cares for many or all of these subsets of patients over the pediatric age range. Another unique factor in the
PICU is that different aged patients will have different incidence patterns of the various types of hospital-acquired infections. For children <5 years of age, the top three nosocomial infections are bloodstream infections (BSIs), pneumonia, and urinary tract infections (UTIs). In children between 5 and 12 years of age, the top three acquired infections are pneumonia, BSIs, and UTIs. In the adolescent population, BSIs are followed by UTIs and then pneumonia in incidence. The location of the hospital plays a role as well with an increased risk of nosocomial infection being noted in developing nations. All types of nosocomial infections when standardized to device days were increased in the ICUs of developing nations in a study by Rosenthal et al. The device utilization rates were noted to be similar in the developed and developing nation ICUs, making the increased infection rate more likely to be secondary to factors within the ICUs, hospitals, or national healthcare systems (
3,
4)
(Table 92.1).
General Risk Factors
In addition to the location within the hospital and the type of
ICU, there are general risk factors that are independent of the type of nosocomial infection. Although these risk factors can influence the likelihood of contracting a nosocomial infection, only a few can be realistically altered to impact nosocomial infection rates.
The age of the patient can affect the risk of nosocomial infection. Younger children, particularly neonates, have the highest risk in the pediatric population. The relative immaturity of the immune system at this point of life coupled with common
ICU interventions, which bypass the physical barriers to infections such as skin and mucosal surfaces, is responsible for the increased risk. The use of parenteral nutrition with high glucose concentrations and lipids is an additional risk factor for infection. Premature infants are impacted the most by these factors, which explain why neonatal ICUs (NICUs) have higher nosocomial infection rates than PICUs. Patients who are immunosuppressed from chemotherapy, human immunodeficiency virus infection, or steroid use are similarly at an increased risk for developing a nosocomial infection.
Severity of illness as predicted by the Pediatric Risk of Mortality (
PRISM) score has been correlated to risk of nosocomial infections. In a study by Arantes et al., a
PRISM score above
13 predicted nosocomial infection in a Brazilian
PICU with 78.9% sensitivity, 64.4% specificity, 21.8% positive predictive value, and 96.1% negative predictive value. Independent risk factors of developing a nosocomial infection were length of stay, prior antimicrobial therapy, and device utilization ratios, with the latter two being the best predictors of nosocomial infection risk (
5).
Understaffing is an independent risk factor for acquiring nosocomial infections. This is most likely owing to the fact that adherence to good hand hygiene has been shown to be inversely correlated to workload. This has been noted for BSIs and ventilator-associated pneumonia (
VAP). Understaffing increases workload and therefore nosocomial infection risk. In a study of
US NICUs, understaffing by 0.11 of a nurse per infant increased the risk of infection from 9% to 14%. Understaffing by 0.22 nurses per infant increased the risk to 21%
(Fig. 92.1). Simply increasing staffing with temporary staff does not reverse this trend. This is likely due to disruption of the normal communication within the interdisciplinary team and familiarity with best practices (
6,
7,
8).
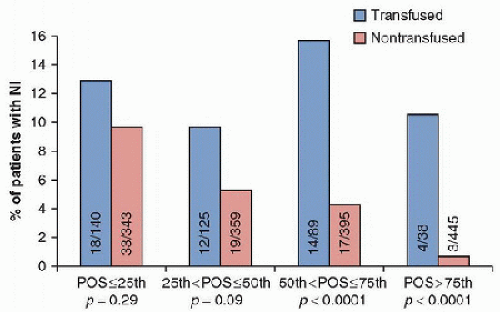
Red blood cell transfusions have been found to be an independent risk factor for the development of nosocomial infections in critically ill adult
ICU patients. In a single-center prospective, observational study, the incidence of nosocomial infection was 14.3% in transfused patients and 5.8% in nontransfused patients. Each unit of packed red blood cells administered increased the risk of developing a nosocomial infection by 9.7%. Increasing severity of illness did not affect the risk of developing a nosocomial infection. However, within each quartile of probability of survival, the transfused group had a higher rate of nosocomial infection, which was significant in all but the most severely ill patients with a probability of survival <25%. Those patients with >25% probability of survival had higher mortality rates, longer
ICU stays, and longer hospitalizations compared to nontransfused patients. A pediatric study also found the increased risk of nosocomial infection associated with transfusion. However, a dose response was not seen with increasing number of units transfused, but mortality was greater in those receiving three or more units (
9,
10)
(Fig. 92.2).
Isolation Precautions

Prevention of nosocomial illness can be, in large part, facilitated through the use of isolation precautions. These precautions can be divided into two categories: standard and transmission-based precautions.
Standard precautions should be used at all times and are designed to prevent the practitioner from coming in contact with potentially infectious bodily fluids. The most important standard precaution is hand hygiene. Soap and water hand washing is considered the gold standard. Use of waterless antiseptic agents is appropriate unless there is the presence of visible dirt, proteinaceous bodily fluids such as blood, or when contamination with spores is likely. Soap and water are necessary under these circumstances. Hand hygiene must be done both before and after patient contact even if gloves are worn. Barriers such as gloves, masks, eye protection, and nonsterile gowns should be worn when contact with bodily fluids or secretions are likely.
Transmission-based precautions are aimed at protection against transmission of infectious organisms from patients with documented or suspected infection as well as those colonized with specific organisms. These additional precautions are over and above the standard precautions and are based on route of transmission: contact, droplet, and airborne transmission. Common organisms requiring each type of isolation are listed in
Table 92.2.
Contact precautions are used for a wide variety of organisms that spread by direct contact with the patient or indirect contact via fomites such as toys, stethoscopes, and unwashed hands. Contact isolation should include singlepatient rooms or cohorting, gowns, and gloves in addition to standard precautions.
Droplet precautions are used for organisms that spread short distances, <3 feet away, from the patient via coughing or sneezing. Droplet isolation includes single-patient rooms or cohorting of patients with the same organism. Healthcare providers should wear a mask with an eye shield in addition to following standard precautions. Some organisms such as adenovirus and influenza require both contact and droplet precautions.
Airborne precautions include additional safeguards to be taken for organisms transmitted by air currents such as tuberculosis, measles, and varicella. Patients should be in private rooms with negative air flow. For measles and varicella isolation, susceptible healthcare providers should avoid contact if possible. For other organisms requiring airborne precautions, a fitted respirator should be worn while in the patient’s room. Isolation should be based on the clinical symptoms or conditions present at admission and should always begin even before the organism is isolated (
11)
(Table 92.3).
For protection against airborne infections, the selection of the correct type of respirator and having a proper fit are crucial. Respirators can be either air-supplying or air-purifying. The air-supplying respirators provide the greatest protection but are expensive and require high amounts of maintenance to assure proper functioning. Air-purifying respirators filter air through a cartridge, which must be selected based on the type of hazard (bacterial or chemical) to be exposed to. These
respirators are protective but not to the same degree as the air-supplying devices. Disposable respirators are air-purifying or filtering devices. The N95 respirators are the most commonly used ones in healthcare settings. The letter designates the mask’s reaction to oil. N means not oil proof. If exposed to oil, the filtering efficiency of the mask may not be maintained. There are also oil-resistant masks and oil-proof masks, designated R and P, respectively. The number indicates the filtering efficiency of the mask with an adequate seal. The number 95 identifies the mask as having the ability to filter at least 95% of particles with a median diameter of 0.3
µm or greater. Most respirators have limitations when used by individuals with facial hair, and specialized devices may be necessary (
12).
 Adherence to infection-control practices, including hand hygiene, is still one of the most beneficial and unfortunately overlooked methods to prevent nosocomial infections.
Adherence to infection-control practices, including hand hygiene, is still one of the most beneficial and unfortunately overlooked methods to prevent nosocomial infections. Isolation precautions are critical to the prevention of transmission of infections within the hospital and need to be put in place at the time of admission based on the likely pathogenic organisms or disease process.
Isolation precautions are critical to the prevention of transmission of infections within the hospital and need to be put in place at the time of admission based on the likely pathogenic organisms or disease process. Surveillance cultures at the time of admission can decrease nosocomial spread of resistant organisms and should be considered if the infection rate or carrier state in the community is high.
Surveillance cultures at the time of admission can decrease nosocomial spread of resistant organisms and should be considered if the infection rate or carrier state in the community is high. Prevention of catheter-related bloodstream infections (CRBSIs) begins at the time of placement with adherence to sterile technique. Care of the catheter site and use of antiseptic- or antibiotic-impregnated catheters can further decrease the risk of infection.
Prevention of catheter-related bloodstream infections (CRBSIs) begins at the time of placement with adherence to sterile technique. Care of the catheter site and use of antiseptic- or antibiotic-impregnated catheters can further decrease the risk of infection. In discussing nosocomial infections, it is important to have an understanding of several definitions. A nosocomial infection is any infection a patient acquires within the hospital setting, which is not present at admission. Even if symptoms develop after discharge, such infections should be considered nosocomial. Community-acquired infections are those present at the time of admission even if they do not necessarily cause symptoms at the time of admission. The differentiation between community-acquired and nosocomial infections may take some investigation and is dependent on incubation times as well as ancillary testing such as sensitivities and genetic typing of the organism. Surgical site infections are associated with the surgical procedure itself, including the wound and the direct surgical field exposed during the procedure. Other infections at distant sites, even though the surgical procedure or anesthesia for the procedure put the patient at risk for such an infection, are considered nosocomial but not surgical site infections. Colonization is the growth or presence of potentially infectious organisms in a cavity, on a surface, in tissue, in body fluids, or even associated with a medical device without causing a host reaction, a clinically adverse event, or disease.
In discussing nosocomial infections, it is important to have an understanding of several definitions. A nosocomial infection is any infection a patient acquires within the hospital setting, which is not present at admission. Even if symptoms develop after discharge, such infections should be considered nosocomial. Community-acquired infections are those present at the time of admission even if they do not necessarily cause symptoms at the time of admission. The differentiation between community-acquired and nosocomial infections may take some investigation and is dependent on incubation times as well as ancillary testing such as sensitivities and genetic typing of the organism. Surgical site infections are associated with the surgical procedure itself, including the wound and the direct surgical field exposed during the procedure. Other infections at distant sites, even though the surgical procedure or anesthesia for the procedure put the patient at risk for such an infection, are considered nosocomial but not surgical site infections. Colonization is the growth or presence of potentially infectious organisms in a cavity, on a surface, in tissue, in body fluids, or even associated with a medical device without causing a host reaction, a clinically adverse event, or disease.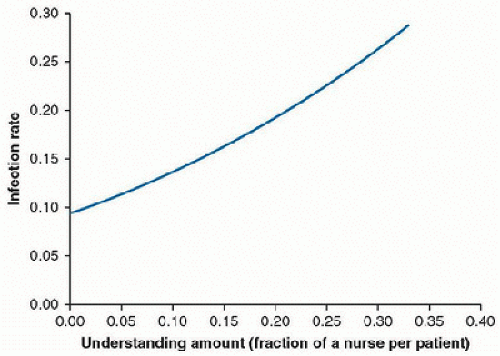
 Prevention of nosocomial illness can be, in large part, facilitated through the use of isolation precautions. These precautions can be divided into two categories: standard and transmission-based precautions. Standard precautions should be used at all times and are designed to prevent the practitioner from coming in contact with potentially infectious bodily fluids. The most important standard precaution is hand hygiene. Soap and water hand washing is considered the gold standard. Use of waterless antiseptic agents is appropriate unless there is the presence of visible dirt, proteinaceous bodily fluids such as blood, or when contamination with spores is likely. Soap and water are necessary under these circumstances. Hand hygiene must be done both before and after patient contact even if gloves are worn. Barriers such as gloves, masks, eye protection, and nonsterile gowns should be worn when contact with bodily fluids or secretions are likely.
Prevention of nosocomial illness can be, in large part, facilitated through the use of isolation precautions. These precautions can be divided into two categories: standard and transmission-based precautions. Standard precautions should be used at all times and are designed to prevent the practitioner from coming in contact with potentially infectious bodily fluids. The most important standard precaution is hand hygiene. Soap and water hand washing is considered the gold standard. Use of waterless antiseptic agents is appropriate unless there is the presence of visible dirt, proteinaceous bodily fluids such as blood, or when contamination with spores is likely. Soap and water are necessary under these circumstances. Hand hygiene must be done both before and after patient contact even if gloves are worn. Barriers such as gloves, masks, eye protection, and nonsterile gowns should be worn when contact with bodily fluids or secretions are likely.





