Fig. 2.1
Mechanisms contributing to peripheral sensitization
At the cord level, many interactions can lead to sensitization, both in terms of functional and structural changes [21]. Voltage-gated calcium channels (VGCC) [22, 23], transient receptor potential vanilloid type 1 (TRPV1) receptors [24, 25], glutamate [7, 26–29], protein kinases [30, 31], serotonin, and N-methyl-d-aspartate (NMDA) [32] have all been described in functional roles within both the spinal cord and brain for sensitization (Figs. 2.1 and 2.2).
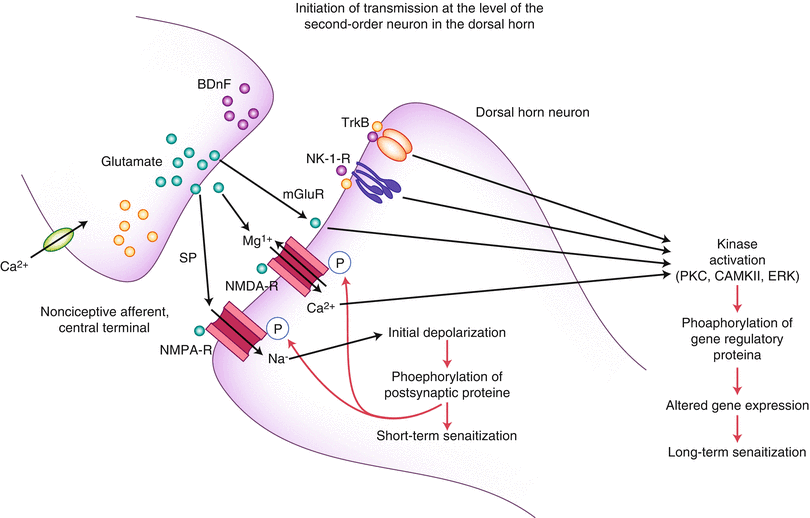

Fig. 2.2
Initiation of transmission at the level of the second-order neuron in the dorsal horn
Structural and functional changes also occur in the spinal cord level regarding astrogliosis, and this effect seems to be mediated through secretion of diffusible transmitters, such as interleukins, ATP, and nitric oxide. The glial cells, via glutamate release, are thought to sensitize second-order neurons. However, they may also have a direct effect via the astrocytic networks that can transduce signals intrinsically [33, 34]. Additionally, animal studies have demonstrated an increase in the number of synapses within the dorsal horn in neuropathic pain [35].
Within the brain, reduced opioid neurotransmission has been noted in animal studies of spinal LTP, especially in brain areas associated with pain modulation and affective-emotional response [36]. LTP in the hippocampus is also involved with memory [37] and fear changes [38]. Spatiotemporal hippocampal changes have been observed in response to persistent nociception [39]. Tetanic stimulation of the ACC increased neurons in the central lateral nucleus of the medial thalamus [40]. fMRI, magnetoencephalography (MEG), positron emission tomography (PET), and voxel-based morphometry (VBM) studies in neuropathic pain have also shown reorganization of cortical somatotropic maps in sensory and motor areas, increased activity in nociceptive areas, recruitment of new cortical areas usually not activated by nociceptive stimuli, aberrant brain behavior normally involved with descending inhibitory pathways, changes in excitatory and inhibitory transmitter systems, and significant structural changes of neurodegeneration (use it or lose it) [41, 42]. These changes have been noted in phantom pain, chronic back pain, irritable bowel syndrome (IBS), fibromyalgia (FM), and two types of headaches. The alterations were different for each pain syndrome but overlapped in the cingulated cortex, the orbitofrontal cortex, the insula, and the dorsal pons [43]. These recent studies support an earlier proposition that the syndromes of chronic daily headache, chronic depression, IBS, and FM may all be different “phenotypes” of one “genotype” secondary to central sensitization [44]. Also, in post-spinal cord injury-related neuropathic pain, VBM studies have shown anatomical changes in pain-related and classic reward circuitry, including the nucleus accumbens, orbitofrontal, dorsolateral prefrontal, and posterior parietal cortices, and the right posterior parietal cortex projected to most of these affected areas [45]. Emotional-affective and cognitive dimensions of pain seem to also demonstrate structural and functional changes in maldynia, especially amygdala and prefrontal cortical areas [46, 47]. In fact, animal models for the amygdala-medial prefrontal cortex-driven pain-related cognitive deficits, including decision making, demonstrated that the cortical deactivation resulted from a shift of balance between excitatory and inhibitory transmission [47]. Hormones may have a role in neuroplasticity as well. Changes in neuroactive steroids during the estrous cycle have been shown to affect GABA-A receptor expression in female rats, resulting in an upregulation of GABA-A receptors late diestrus causing an increased excitability of output neurons in the periaqueductal gray (PAG) and clinically resulting in hyperalgesia [48].
Hence, we now have seen a neuroplastic transformation within the nervous system from a marvelous eudynic pain transmission model to one which demonstrates anatomic cellular changes, chemical transmitter and neuromodulator changes, and physiological functional changes via the process of sensitization. Indeed, our pain train now has different numbers of cars, different speeds at which to travel, changes in the stations along the way, and different tracks upon which it must now travel. It is what it has become!
Neuroplastically Remodeled Pain System
Persistent Pain: The “Ugly”
We will now turn more toward the clinical side of neuroplasticity and look at some specific pain problems. Ultimately, we will look at what can be done about them to ease our patient’s suffering.
Limbically Augmented Pain Syndrome (LAPS) [49]
LAPS was described in a seminal pain paper to account for people who demonstrate more pain and pain behaviors than would be expected based solely on physical findings. These patients had previously been labeled as “hysterics,” “crocks,” and malingerers. Their presentation was usually affectively colorful, intense, and consisted of dramatic levels of dysfunction based on what previously looked like very little wrong physically. Through a very extensive comparison of clinical symptoms to sensitization research work, this paper clarified the role of central sensitization in both the traditional pain pathways and non-pain pathways regarding the affective-motivational and cognitive dimensions to pain perception. LAPS provides a foundational understanding of how sensitization presents clinically and why the primary pain and secondary non-pain complaints make sense. This includes many maldynia-accompanying complaints such as memory problems, slower thinking, non-restful sleep, decreased energy, lack of drive, decreased mental concentration and focus, anxiety, depression, anger, irritability, social isolation, a marked change in self-perception, and other frequent complaints we hear from our patients. The neuroplastic changes secondary to sensitization which account for decreased pain threshold, increased pain perception, recruitment, amplification of pain signaling within the central nervous system, and the intertwined role of pain- and non-pain-related inputs to pain have all been borne out and clarified through the research that has progressed since LAPS was identified, primarily due to improved technologies, but based on the core principles presented in the LAPS paper.
Through the LAPS foundation, we can make more sense out of the two dimensions of pain: the sensory-discriminative and affective-motivational dimensions described by Price [8]. Understanding these two dimensions and the overlapping brain functions involved with both helps unify our understanding of a person’s pain perception, again reminding us of Osler’s decree: one person, one disease. Rather than separate the two dimensions of pain and their respectively related symptoms, we can now focus on one person with all those complaints related to alterations in neuroplastic changes of the brain.
Fibromyalgia
Clinical aspects of neuroplastic changes in fibromyalgia are easily delineated, including many of those identified by the LAPS paper. However, peripheral evidence of change is basically lacking, in spite of much research on the peripheral tissues and peripheral pain transmissions. Within the central nervous system, however, much has been demonstrated. Fibromyalgia sufferers demonstrate hyperalgesia and allodynia, and this seems to result from an abnormal temporal summation of pain [50]. Changes in both sensory and motor brain have been found. One MRI and VBM study showed decreases in gray matter in the right superior temporal gyrus and left posterior thalamus, with increased gray matter in the left orbitofrontal cortex, left cerebellum, and striatum [51]. Another study found increased levels of serum BDNF in 30 female fibromyalgia patients, with no correlation to age, disease duration, pain score, number of pain tender points, or depression rating scores (HAM-D) [52].
Some of the treatments for fibromyalgia include pharmacological approaches designed to lower the level of pain transmission secondary to the neuroplastic sensitization by “calming down” the system at spinal and brain levels (see Part I. Medical Approaches). Other treatments include mind-body paradigms, as well as physical modalities. One study demonstrated benefit with a mind-body treatment utilizing psychosocial genomic postulates coupled with ideodynamic hand movements [53]. One study is being planned to utilize virtual exercise for those fibromyalgia patients who tend to avoid exercise as part of a catastrophizing style [54]. Moderate exercise for 24 weeks has been shown to have benefit for those able to tolerate it, resulting in improved health status and quality of life [55]. A separate 10-week exercise study demonstrated reduction in anxiety, improved sleep, and improved quality of life [56]. However, a group who had demonstrated improved daily step count by 54 %, improved functioning by 18 %, and reduced pain by 54 % in a 12-week trial found poor sustainability at 12-month follow-up, at which time the patients did not differ from controls on pain, physical activity, tenderness, fatigue, depression, the 6-min walk test, or self-reported functioning [57]. These findings would raise question as to what could be done to maintain the home treatment strategies long enough, or what could be added to them, in order to establish positive neuroplastic changes (i.e., what fires together wires together and what fires apart, wires apart).
Phantom Pain
Phantom limb sensations and pain have become more prominent in the literature since the incidence of amputation has increased as a result of the recent wars throughout the world [58]. The most recent successful treatments for phantom pain have also been based on what can change the brain LTP, hence, utilizing neuroplasticity to understand the pathophysiology of phantom pain and also to reverse it.
Studies have shown changes in the cortical representation of the affected limb and a correlation between these changes and the phantom pain. Mechanisms for the phantom pain are thought to relate to a loss of gamma-amino-butyric acid (GABA)-ergic inhibition, glutamate-mediated LTP changes, and structural changes such as axonal sprouting, and furthermore, these changes and consequent pain seem to be more extensive if chronic pain precedes the amputation [59].
One proposal suggests the imbalance of the system to be part of the problem. Specifically, the motor cortical body-representation cells involute, while the sensory cortical body-representation cells remain, the resulting imbalance producing the phantom pain. Reconciliation of this imbalance produces relief [60]. In fact, one treatment protocol utilizing imagined amputated limb movement coupled with existing counterpart limb movement resulted in fMRI evidence of elimination of the cortical reorganization and a reduction in constant pain and exacerbation pain [61]. Eye movement desensitization and reprocessing (EMDR) has also been shown to virtually eliminate phantom pain, without return barring further trauma to the body, through a similar process of alternating sensory input coupled with mental processing of the phantom pain [62, 63].
Complex Regional Pain Syndrome (CRPS)
CRPS is currently understood to be a complex of altered somatosensory, motor, autonomic, and inflammatory systems. But the central feature is both peripheral and central sensitization. Especially important to this sensitization is the neuroplastic alterations in the dorsal horn of postsynaptic NMDA receptors via chronic C-fiber input, among other changes. Motor changes are effected by calcitonin gene-related peptide (CGRP), substance P, and pro-inflammatory cytokines involved in the inflammatory process. Recent evidence implicates sensitization of adrenergic receptors in the sympathetic system having an influence on the C-fibers [64]. In animal studies, chronic peripheral inflammation has been shown to increase AMPA receptor-mediated glutamergic transmission in the ACC, which then increases the central excitatory transmission [65].
Visceral Pain
Visceral pain drives many doctor visits by patients, and it is one of the most common complaints in primary care offices. Visceral afferents have been found to play a role in tissue homeostasis by monitoring the viscera and contributing efferent functions via the release of small molecules such as CGRP that can drive inflammation. These afferents are highly plastic and are responsive to their cellular environment. They are quite susceptible to long-term changes associated with irritable bowel syndrome (IBS), pancreatitis, and visceral cancers [66]. In fact, recent work on chronic pancreatitis links sensitization to this syndrome, with descriptions of temporal and spatial alterations of intrapancreatic nerves and central neuroplastic consequences [67]. This process in chronic pancreatitis, then, seems to involve peripheral nociception, peripheral pancreatic neuropathy and neuroplasticity, and central neuroplastic changes as follows: sustained sensitization of pancreatic peripheral nociceptors by neurotransmitters and neurotrophic factors following neural damage, resulting in intrapancreatic autonomic “neural remodeling,” which in turn causes our familiar hyperexcitability of second-order dorsal horn neurons, followed by viscerosensory cortical spatial reorganization [68].
Headache
Much work has been done regarding sensitization involving headache [69]. Recent understanding of sensitization in migraine considers peripheral sensitization leading to intracranial hypersensitivity (worsening the headache with cough and activity) and sensitized neurons becoming hyperresponsive to normally innocuous and unperceived fluctuations in intracranial pressure changes from arterial pulsation, resulting in the throbbing sensation. Central sensitization results in hyperexcitability of second-order neurons in the trigeminocervical complex, again a result of increased glutamate sensitivity of NMDA receptors and neuronal nitric oxide synthase activity. Clinically, this is manifested by facial and scalp allodynia along with neck stiffness [70].
Additionally, in chronic posttraumatic headache, a VBM study found spatial cortical reorganization to include decreased gray matter in the ACC and dorsolateral prefrontal cortex after 3 months. After resolution of the headache, at 1-year follow-up, patients who had developed the chronic headaches also showed an increase in gray matter in antinociceptive brainstem centers, thalamus, and cerebellum [71].
Postsurgical Pain
Chronic postoperative pain is becoming more prominently recognized. It is again thought to follow sensitization of the peripheral and central system by persistent acute postoperative pain. In most patients, it resembles neuropathic pain and occasionally follows continuous post-op inflammation [72]. This problem is estimated at between 10 and 80 %, and increased risk is associated with the existence of preoperative pain, the intensity and duration of post-op pain, and the type of surgery (high-risk surgeries) such as thoracotomy, breast, inguinal herniorrhaphy, and amputations [73]. The use of perioperative regional anesthesia has been shown to reduce the incidence, compared with intravenous morphine [74].
What to Do About It: Retraining the Brain
Multiple treatment approaches have been investigated for persistent pain, some designed to reduce the pain, others to improve functional status, and still others to reestablish a sense of contentment with life. The main theme behind all treatments is to try to establish a reversal of the neuroplastic sensitization or find ways to diminish its significance [75, 76].
Low back pain has been improved utilizing a training program model of delayed postural activation of the deep abdominal muscle, the transverse abdominis (TrA). Motor skill training induced an anterior and medial shift in motor cortical representation of the TrA, more closely resembling that of healthy persons. This training reversed the neuroplastic reorganization associated with chronic low back pain [77]. Other paradigms for chronic musculoskeletal pain also identify the need for motor learning as an important component for success, secondary to their ability at cortical reorganization [78]. One example is the use of peripheral electrical stimulation, which has been shown to develop rapid plastic change in the motor cortex, with parameters of variation in intensity of stimulation and longer periods of stimulation having the most sustained effects [79]. A fMRI study involving low-frequency electrical stimulation of cutaneous afferents in healthy volunteers resulted in pain relief and increased activity in the ACC, anterior insula, striatum, and frontal and temporal cortices, demonstrating long-term depression (LTD) of pain-related cerebral activation involving sensory, affective, cognitive, and attentional processes [80].
Acupuncture (see Chap. 10 for a detailed analysis of the approach) and massage have also been found to be peripheral stimulations which can cause central reorganizations. This has been described in terms of changing the neuroplastic adaptations associated with pain and addictive disorders [81].
Other therapeutic approaches include training of perceptual abilities, motor function, direct cortical stimulation, and behavioral approaches. Treatments that combine several modalities, such as imagery, mirror treatment, and prostheses, have been shown to have benefit [82]. For example, mirror therapy has been utilized for phantom pain, hemiparesis from stroke, and CPRS [83]. A somatosensory evoked potential (SEP) study involving chiropractic manipulation of the neck in subjects without current pain, but a history of chronic cervical pain, suggested alteration of the cortical integration of dual somatosensory input [84]. Paired associative stimulation in which peripheral nerve stimulation is followed by transcranial magnetic stimulation resulted in increased volleys of the descending inhibitory pathways [85], resulting in apparent LTD [86]. Even such techniques such as caloric restriction, via reduced intake of calories or intermittent fasting, have been shown to stimulate neurogenesis, enhance plasticity affecting pain sensation, cognitive function, and possibly resist brain aging. This is felt to occur through neurotrophic factors, neurotransmitter receptors, protein chaperones, and mitochondrial biosynthesis regulators which contribute to stimulation of the neuronal plasticity and resistance to oxidative metabolic insults [87]. Interestingly, one of the three worldwide characteristics of people who live the longest is eating 25 % less than the rest of their community members (caloric restriction) [4]. Cognitive-behavioral approaches have included somatosensory amplification associated with training in affect differentiation and the interaction of somatoform pain and interpersonal relationships [88].
It has been the clinical experience of the current author that the most effective treatment for maldynia or maldynia-eudynia combinations is a comprehensive approach designed to retrain the brain. This includes the utilization of some/all of the below modalities, individualized for each patient, such as myofascial release, unwinding, movement, electrical stimulations, muscle and ligament injections, exercising, postural training, guided imagery, body manipulations, visualization, meditation, spiritual healing, energy work, hypnosis, use of appropriate medications, inappropriate medication reductions, low-glycemic load nutritional approaches, nutritional supplements, bioidentical hormones, aroma therapy, graduated functional increases, massage and therapeutic touch, acupuncture, cognitive-behavioral treatment, Eye Movement Desensitization and Reprogramming (EMDR), NeuroEmotional Technique (NET), music therapy, family therapy and education, and patient education including neuroplasticity and brain function and how they are affecting their lives. The author of this chapter also utilizes written materials, some self-created and some published. Patients are referred to such items such as the American Pain Foundation (APF) website, the American Chronic Pain Association (ACPA) website, The Brain That Changes Itself [5], Rewire Your Brain [3], The Mind and the Brain [4], You Can Heal Your Life [89], The Quantum Brain [90], Power versus Force [91], and Healing and Recovery [92], among others.
These various therapies, educational materials, and philosophical approaches are often delivered individually, but sometimes simultaneously in a dual stimulatory approach through co-treatment (i.e., two therapists utilizing different treatments simultaneously, often incorporating two different senses/physical modalities at the same time). Treatments such as EMDR and NET incorporate two types of brain activity within one treatment approach. Some of these modalities take time and ritualistic practice in order to make neuroplastic changes (LTD, depotentiation of the LTP that occurred, or new LTD which is positive), while others, such as EMDR and NET, can have a rapid and lasting response, implying a neuroplastic reorganization that is immediate. Most of the therapeutic approaches mentioned above, however, must be done extensively and relatively frequently to make cortical changes permanently. We view ALL of these possible treatment modalities to have the same ultimate goal: RETRAINING THE BRAIN!
Summary
In this chapter, we have discussed the concept of neuroplasticity as the operating system of our nervous system computer. Without judgment, neuroplasticity can be of extreme usefulness, or it can produce, via sensitization, an “altered computer program” with devastating life effects of pain and suffering.
We have looked at the normal neuroplastic pain system for eudynia and what can go awry to result in maldynia, a disease. We have seen how neuroplasticity and sensitization can account for the good, the bad, and the ugly for pain transmission.
Although the mechanisms involved with neuroplastic sensitization of peripheral and central nervous components have been studied and continue to be researched, we still do not know which is the chicken or the egg. Exactly why this sensitization occurs, and who are the vulnerable people, is still a mystery. We do know that the brain can undergo neuroplastic reorganization from lower level (peripheral and spinal) changes or from higher level (mind) inputs. The system remains plastic throughout time and is responsive to inputs from any level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






