Neurophysiologic Basis of Pain
Gary Jay Brenner
Severe pain is world destroying.
—Elaine Scarry from The Body in Pain
One of the most important functions of the nervous system is to provide information about potential and actual bodily injury. Nearly a century ago, in 1906, Sir Charles Sherrington defined nociception as the sensory detection of a noxious event or a potentially harmful environmental stimulus. He explicitly distinguished nociception from pain, a complex human experience that involves sensory, psychologic, and cognitive components. Pain is currently defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.” The pain system may be grossly divided into the following components:
Nociceptors, the specialized receptors in the peripheral nervous system that detect noxious stimuli, primary nociceptive afferent fibers, normally Aδ and C fibers, which transmit information about noxious stimuli to the dorsal horn of the spinal cord
Ascending nociceptive tracts, for example, the spinothalamic and spinohypothalamic tract (SHT), which convey nociceptive
stimuli from the dorsal horn of the spinal cord to higher centers in the central nervous system (CNS)
Higher centers in the CNS that are involved in discrimination of pain, affective components of pain, memory components of pain, and motor control related to the immediate aversive response to painful stimuli
Descending systems that allow higher centers of the CNS to modify nociceptive information at multiple levels
I. NOCICEPTORS
1. Definitions
Although somewhat confusing, the term nociceptor is used to refer both to the free nerve terminals of primary afferent fibers that respond to painful, potentially injurious stimuli and to the entire apparatus (sensory neuron, including free terminals) capable of transducing and transmitting information about noxious stimuli. In this chapter, the term nociceptor is used to refer to the entire nociceptive primary afferent.
Free nerve terminals contain receptors capable of transducing chemical, mechanical, and thermal signals. Recently, for example, a membrane receptor that responds to noxious heat has been cloned (it has been designated TRPV1), and, interestingly, this receptor is also stimulated by capsacin, the molecule responsible for the “hot” sensation associated with hot peppers. Nociceptive terminals innervate a wide variety of tissues and are present in both somatic and visceral structures including the cornea, tooth pulp, muscles, joints, respiratory system, cardiovascular system, digestive system, urogenital system, and meninges, as well as skin.
Nociceptors may be divided according to three criteria: degree of myelination, type(s) of stimulation that evokes a response, and response characteristics. There are two basic classes of nociceptors based on their degree of myelination and conduction velocity. A-delta fibers (Aδ) are thinly myelinated and conduct at a velocity of 2 to 30 m per second. C fibers are unmyelinated and conduct at a velocity of less than 2 m per second (see Table 1). Aδ and C nociceptors can be further divided according to the stimuli they sense. These nociceptors may respond to mechanical, chemical, or thermal (heat and cold) stimuli, or a combination (polymodal). For example, C-fiber mechanoheat receptors respond to noxious mechanical stimuli and intermediate heat stimuli (41°C to 49°C), have a slow conduction velocity, and constitute most nociceptive afferent fibers. Aδ mechanoheat receptors can be divided into two subtypes. Type I receptors have a high heat threshold (>53°C) and conduct at relatively fast velocities (30 to 55 m per second). These receptors detect pain during high-intensity heat responses. Type II receptors have a lower heat threshold and conduct at slower velocity (15 m per second). Some receptors respond to both warmth and thermal pain. Some C and Aδ fibers are mechanically insensitive but respond to heat, cold, or a variety of chemicals (e.g., bradykinin, hydrogen ions, serotonin, histamine, arachidonic acid, and prostacyclin).
Table 1. Classification of fibers in peripheral nerves | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
2. Primary Afferent Fibers
The neural impulses originating from the free endings of nociceptors are transmitted via primary afferent nerves to the spinal cord, or, if originating from the head and neck, the impulses are transmitted via cranial nerves to the brainstem. Most primary afferent fibers of innervating tissues below the head have cell bodies located in the dorsal root ganglion (DRG) of spinal nerves. Primary afferent fibers of cranial nerves V, VII, IX, and X (the sensory cranial nerves) have cell bodies in their respective sensory ganglia.
Most nociceptors are C fibers, and 80% to 90% of C fibers respond to nociceptive input. The differences in conduction velocities and response characteristics of Aδ and C fibers may explain the typical subjective experience of pain associated with a noxious stimulus: a first pain (so-called epicritic pain) that is rapid, well localized, and pricking in character (Aδ), followed by a second pain (so-called protopathic pain) that is burning and diffuse (C fiber). Visceral afferent nociceptive fibers (Aδ and C) travel with sympathetic and parasympathetic fibers; their cell bodies are also found in the DRG. Muscle is also innervated by both Aδ and C fibers, and, interestingly, muscle pain appears to be limited in quality to that of a cramp.
3. Dorsal Horn Synapses and Biochemical Mediators
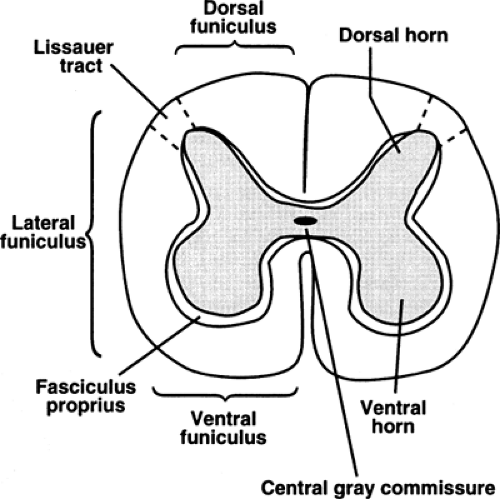
Primary afferent nociceptors enter the spinal cord via Lissauer tract and synapse on neurons in the dorsal horn (see Fig. 1). Lissauer tract is a bundle of predominantly (80%) primary afferent fibers, consisting mainly of Aδ and C fibers that penetrate the spinal cord en route to the dorsal horn. After entering the spinal cord, Aδ and C fibers run up or down one or two segments before synapsing with second-order neurons in the dorsal horn. The dorsal horn synapse is an important site of further processing and integration of the incoming nociceptive information. The dorsal horn may be a point at which nociceptive information is conducted to higher centers, or a point at which nociceptive information is inhibited by descending systems. The responsiveness of dorsal horn neurons may change in response to prior noxious afferent input, particularly repetitive input (central sensitization).
Biochemical Mediators
Numerous neurotransmitters and other biochemical mediators are released in the dorsal horn. These substances are derived from three main sources:
primary afferent fibers
interneurons
descending fiber systems
The neurochemistry of the dorsal horn is complicated, and there are qualitative differences between the pharmacology of acute pain and that of the facilitated pain states associated with
chronic noxious stimulation. Some of the neurochemical mediators can be categorized as excitatory or inhibitory, although many serve complex and mixed functions. For example, the endogenous opioid dynorphin may be inhibitory or excitatory depending on the state of the nervous system. The following are examples of excitatory and inhibitory substances active in the dorsal horn.
chronic noxious stimulation. Some of the neurochemical mediators can be categorized as excitatory or inhibitory, although many serve complex and mixed functions. For example, the endogenous opioid dynorphin may be inhibitory or excitatory depending on the state of the nervous system. The following are examples of excitatory and inhibitory substances active in the dorsal horn.
Excitatory Neuromediators
The excitatory amino acids glutamate and aspartate
The neuropeptides substance P (SP) and calcitonin gene–related peptide (CGRP)
Growth factor brain-derived neurotrophic factor (BDNF)
Bradykinin
Inhibitory Neuromediators
Endogenous opioids such as enkephalin and β-endorphin
γ-aminobutyric acid (GABA)
Glycine
Cells of the dorsal horn possess specific receptors for the substances listed earlier, as well as receptors for a multitude of other neurochemicals (some probably undiscovered). Of particular note is the receptor for glutamate, the N-methyl-d-aspartate (NMDA) receptor, which is widely distributed in the dorsal horn. There are now extensive experimental data implicating the NMDA receptor in the generation and maintenance of facilitated pain states.
4. Peripheral Sensitization
Prolonged noxious stimulation can sensitize nociceptors. Sensitization refers to a decreased threshold as well as to an increased response to suprathreshold stimulation. It is observed following direct nerve injury and inflammation and is the result of a complex set of transcriptional and posttranslational changes in the primary nociceptive afferents. Sensitization of the entire nociceptive pathway can arise secondary to changes in the CNS (central sensitization) or the periphery (peripheral sensitization). Once sensitization is established, it is often impossible to clinically separate central from peripheral contributions to the process of sensitization. The related topics on hyperalgesia, allodynia, inflammation, and nerve injury are briefly discussed in the following text.
(i) Hyperalgesia
Tissue damage activates nociceptors; if the damage is prolonged and intense, it can generate a state in which there is a lowered threshold to painful stimuli. This state is termed hyperalgesia. In hyperalgesic areas, one can clinically observe an increased response to noxious stimuli. There are alterations in both the subjective and the neurophysiologic responses to stimuli. Thus, a lowered pain threshold and an increased response to painful stimuli are not only subjectively experienced by the patient, but also demonstrated by the nociceptors. Primary hyperalgesia is hyperalgesia at the
site of injury; secondary hyperalgesia refers to hyperalgesia at the site surrounding the injured tissue. Secondary hyperalgesia is thought to reflect changes occurring in the CNS and would therefore be an example of central sensitization.
site of injury; secondary hyperalgesia refers to hyperalgesia at the site surrounding the injured tissue. Secondary hyperalgesia is thought to reflect changes occurring in the CNS and would therefore be an example of central sensitization.
(ii) Allodynia
In addition to developing a lowered threshold for noxious stimuli following tissue damage (hyperalgesia), patients may also suffer a postinjury state in which normally innocuous stimuli are perceived as painful. This phenomenon is termed allodynia. For example, a very light touch in the area of a burn or in an area associated with postherpatic neuralgia can generate excruciating pain. Like hyperalgesia, allodynia is thought to be caused by plastic changes in both primary sensory fibers and spinal cord neurons.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree