Neuropathic Pain Syndromes
Dennis Dey
Anne Louise Oaklander
It is evidently impossible to transmit the impression of pain by teaching, since it is only known to those who have experienced it. Moreover, we are ignorant of each type of pain before we have felt it.
—Galen, 129–199
Neuropathic (or neuralgic) pain is always caused by injury to the pain-sensing portions of the nervous system, although many such injuries do not cause chronic pain. The hallmark of neuropathic pain is that it occurs or persists in the absence of tissue injury or inflammation, and therefore, it lacks the protective benefits of acute pain. Chronic pathologic pain without recovery is more devastating than pain that accompanies tissue injury and resolves with healing. Also, this “invisible pain” often creates a psychological burden because family members and medical providers cannot see an objective correlate. Mutual trust and communication between patient and caregivers must be established for effective care.
Neuropathic pain ensues when pain-processing (nociceptive) neurons generate action potentials despite the absence of tissue injury. Normally, neurons fire only when activated by tissue-damaging stimuli (e.g., high heat, cutting, or inflammation). Neuropathic pain is therefore a nociceptive hallucination analogous to the sensory hallucinations that can occur after damage to other sensory systems (e.g., tinnitus after hearing loss). Unfortunately, the precise location of such misfiring is often unknown, or it may occur at multiple levels of the nervous system, making it difficult to treat the pain. Any type of neural injury—whether from trauma, infection, or inflammation—or biochemical
imbalance can produce neuralgic pain, and interruptions anywhere along the pain pathways, from the nerve endings in the skin to the somatosensory cortices, will suffice. The most common clinical feature is chronic pain paradoxically colocalizing with decreased sensory function. Other neurologic symptoms, such as motor or autonomic disturbances, are common but may go unrecognized. Because there are no objective diagnostic tests for this condition, a careful history and physical examination remain the most important diagnostic tools.
imbalance can produce neuralgic pain, and interruptions anywhere along the pain pathways, from the nerve endings in the skin to the somatosensory cortices, will suffice. The most common clinical feature is chronic pain paradoxically colocalizing with decreased sensory function. Other neurologic symptoms, such as motor or autonomic disturbances, are common but may go unrecognized. Because there are no objective diagnostic tests for this condition, a careful history and physical examination remain the most important diagnostic tools.
I. CLINICAL PRESENTATION
1. Pain and Sensory Abnormalities
The clinical spectrum of neuropathic pain ranges from barely noticeable to severely disabling. One or more of the following symptoms are present in neuropathic pain patients regardless of etiology, mechanism, and location of neural injury:
Ongoing (or stimulus-independent) chronic pain described as “burning,” “aching,” “crushing,” or “gnawing”
Abnormal stimulus-evoked pain, especially after mechanical stimuli
Brief lancinating pains described as brief, severe jolts of pain, sometimes called “electrical” or “lightning pains”; these can be spontaneous or can be evoked by a stimulus; this type of pain does not occur without neural injury and is near-pathognomonic for the neuralgias
The relative importance of these different types of pain varies across diseases and even within the same patient at different times. For instance, trigeminal neuralgia is known for severe lancinating pains, which can be provoked by an innocuous stimulation of a trigger zone on the face. However, questioning will reveal that most patients have also experienced the other features of neuropathic pain at some point.
Perturbations of somatosensory function (see Fig. 1) are also characteristic of neuropathic pain. The most common feature is numbness, or hypoaesthesia. Paradoxically, patients may report decreased sensation in the same area where they experience maximum pain or they may be unaware of their sensory deficit until it is elicited during examination. Pain thresholds can be lowered—hyperalgesia—or raised—hypoalgesia Paresthesias, sometimes described as a pins-and-needles feeling, are positive sensory phenomena suggestive of neuropathic pain. Patients may also use the word numbness to describe paresthesias, so the terminology may require clarification. Some patients report allodynia or pain from innocuous stimuli, such as light touch. Mechanical allodynia is most common, and some patients go to extreme lengths to avoid having their neuropathic area touched (see Fig. 2). Pain can be caused by contact with clothing or bedsheet or even by a breeze. Patients with trigeminal neuralgia may not be able to shave areas of their face, and intraoral allodynia can interfere with eating and cause weight loss or malnutrition. Some women with postherpetic neuralgia (PHN) on the torso are unable to wear a brassiere, and some patients with distal painful neuropathies hang their feet over
the edge of their bed to avoid the bedclothes. Many patients describe worsening of their pain in cold weather. This may reflect a component of cold allodynia. Warmth allodynia prompts some patients to carry ice bags or fans to continually cool the painful area. Quantitative sensory testing has not revealed a consistent pattern of sensory abnormalities that characterizes neuropathic pain.
the edge of their bed to avoid the bedclothes. Many patients describe worsening of their pain in cold weather. This may reflect a component of cold allodynia. Warmth allodynia prompts some patients to carry ice bags or fans to continually cool the painful area. Quantitative sensory testing has not revealed a consistent pattern of sensory abnormalities that characterizes neuropathic pain.
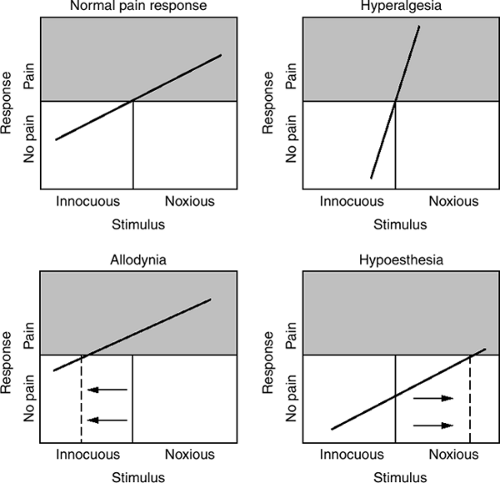 Figure 1. Graphic representation of perturbations of somatosensory function associated with pathologic pain states. |
Some of these symptoms occur in acute and inflammatory/nociceptive pain (e.g., sunburn). This observation suggests that acute pain may have a neuropathic component. However, if the neurons are normal, these symptoms remit once the tissue injury heals, whereas in case of neuropathic pain they persist.
2. Other Clinical Features
Because different types of neurons mingle closely within the central and peripheral nervous systems, the damage affecting nociceptive neurons often affects other neural systems as well. Patients whose motor pathways are damaged can have abnormalities of muscle tone, bulk, and strength. The presence of objective motor signs or an abnormal electromyogram (EMG) can be helpful in making the diagnosis of neuropathic rather than nociceptive pain. However, because the nociceptive neurons are not tested, a normal EMG does not rule out neuropathic pain. Increased tone in the affected area is suggestive of a central
lesion, such as that from stroke; in contrast, peripheral lesions, such as compressive radiculopathies or nerve injuries, can reduce tone. Dystonias, or sustained pathologic cocontraction of agonist and antagonist muscles, are increasingly recognized as a component of complex regional pain syndrome (CRPS). It remains unclear whether these abnormal movements reflect peripheral motor damage from the inciting trauma or whether they are caused by secondary abnormalities in the spinal cord or brain. Sometimes, only minor motor symptoms, such as a tendency to develop muscle cramps, are present. Of course, disuse of a painful limb can cause secondary motor changes (e.g., contractures) as well. Occasionally, the motor damage is primary and pain is secondary to the abnormal muscle contractions, as in the focal, segmental, or generalized primary dystonic syndromes.
lesion, such as that from stroke; in contrast, peripheral lesions, such as compressive radiculopathies or nerve injuries, can reduce tone. Dystonias, or sustained pathologic cocontraction of agonist and antagonist muscles, are increasingly recognized as a component of complex regional pain syndrome (CRPS). It remains unclear whether these abnormal movements reflect peripheral motor damage from the inciting trauma or whether they are caused by secondary abnormalities in the spinal cord or brain. Sometimes, only minor motor symptoms, such as a tendency to develop muscle cramps, are present. Of course, disuse of a painful limb can cause secondary motor changes (e.g., contractures) as well. Occasionally, the motor damage is primary and pain is secondary to the abnormal muscle contractions, as in the focal, segmental, or generalized primary dystonic syndromes.
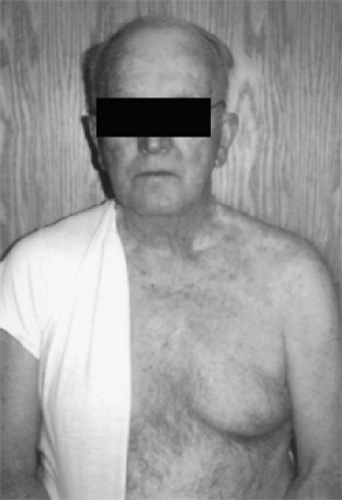 Figure 2. Some patients go to extreme lengths to avoid having their neuropathic area touched. This man has postherpetic neuralgia (PHN) and has cut his shirt in half because of allodynia. |
Autonomic abnormalities are common and are characteristic in several neuralgias because most peripheral nociceptive neurons have efferent autonomic functions as well as afferent actions. Therefore, autonomic abnormalities are to be expected when these neurons are damaged. Damage to these axons produces changes in color and temperature in the affected tissues and can
cause swelling because of abnormal leakage of intravascular fluid. These effects can exacerbate pain. Small-fiber neuropathies are an underrecognized cause of pedal edema. Trigeminal neuralgias are an underrecognized cause of chronic rhinorrhea. Similarly, animal studies have shown clearly that the growth of skin, hair, nails, and other cutaneous structures becomes abnormal if innervation is disrupted. Damage to the autonomic neurons that innervate more centrally located areas can produce symptoms such as orthostatic hypotension, impotence, delayed gastric emptying, abnormal sweating and thermoregulation, and difficulties with elimination. Occasionally, cardiac arrhythmias may become clinically significant after central or peripheral lesions.
cause swelling because of abnormal leakage of intravascular fluid. These effects can exacerbate pain. Small-fiber neuropathies are an underrecognized cause of pedal edema. Trigeminal neuralgias are an underrecognized cause of chronic rhinorrhea. Similarly, animal studies have shown clearly that the growth of skin, hair, nails, and other cutaneous structures becomes abnormal if innervation is disrupted. Damage to the autonomic neurons that innervate more centrally located areas can produce symptoms such as orthostatic hypotension, impotence, delayed gastric emptying, abnormal sweating and thermoregulation, and difficulties with elimination. Occasionally, cardiac arrhythmias may become clinically significant after central or peripheral lesions.
II. MECHANISMS OF NEUROPATHIC PAIN
Neuropathic pain is generated by electrical hyperactivity of neurons along the pain pathways. The sensory pathway consists of at least three neurons, and lesions anywhere along the pathway can lead to neuropathic pain. Changes in expression of neuronal ion channels and receptors, synaptic connectivity, and anatomy all contribute to neuropathic pain (neural plasticity). Although functional changes are related to repetitive painful input into the nociceptive system (central sensitization), neuropathic pain is also associated with anatomical changes. Severe degeneration of peripheral nociceptive neurons has been shown in at least two types of neuralgias, PHN after shingles and peripheral small-fiber neuropathies. Loss of inhibitory spinal interneurons and growth of touch fibers into the pain pathway may occur as well. In phantom limb pain, cortical sensory neurons that have lost their input from the periphery may connect to neighboring nerve cells and lead to bizarre phenomena such as the evocation of phantom hand pain by touching the subject’s mouth.
Increasingly, it has been recognized that nearby nonneuronal cells, including both neural support cells and immunocytes, secrete factors that alter the local macromolecular milieu and that modulate nociceptive signal processing. Finally, limbic and cognitive circuits influence the capacity of the brain to “listen to” incoming nociceptive signals. Acute pain, even when severe, can be suppressed in the presence of other more urgent concerns, such as fight or flight; however, chronic pain can be amplified by emotional states such as anxiety and depression. The impetus for unraveling the complex mechanisms of neuropathic pain is the hope of developing new treatments and of using existing medications to target pain and related symptoms more precisely. Detailed discussion of pain pathways and pain mechanisms can be found in Chapter 1.
III. SPECIFIC NEUROPATHIC PAIN SYNDROMES
1. Peripheral Syndromes
(i) Generalized Painful Polyneuropathies
Most neuralgias originate from peripheral nerve injury. Bilateral pain, usually, but not always, starting in both feet and spreading proximally, usually indicates the presence of a length-dependent polyneuropathy. These polyneuropathies can be classified by
etiology, distribution, and pathology. Some are specific to the type of axon involved, but many affect more than one type of axon (e.g., sensory-motor polyneuropathy). Motor symptoms typically include weakness and affect the distal muscles, often the extensor groups. Sensory disturbances can be classified by examination with pin, heat or cold, and vibratory stimuli. The distribution of neuropathies can be symmetrical or asymmetrical, multifocal or focal. In the polyneuropathies, neuronal dysfunction is first reported in the extremities, the distal portions of the longest axons. Often, the earliest symptoms are those of the autonomic and small-fiber sensory modalities. The diagnostic evaluation includes a thorough history to ascertain the etiology, including questions regarding systemic illnesses, injuries, nutritional deficiencies, family history, and toxin exposure. Lists of recommended diagnostic tests are available at http://www.neuroskinbiopsy.mgh.harvard.edu. Painful sensory neuropathies have been associated with systemic disorders such as human immunodeficiency virus (HIV) infection, diabetes mellitus, alcohol abuse, hepatitis, malignant cancers, monoclonal gammopathies (IgG, IgA, and IgM), rheumatoid arthritis, collagen vascular disease, and amyloidosis. Inherited neuropathies, such as the hereditary sensory and autonomic neuropathies (HSAN), can also cause neuropathic pain. HSAN-1 is the most common characterized HSAN seen in the United States. In severe neuropathies, there can also be complete absence of pain sensation with devastating consequences (mutilation secondary to repetitive and unnoticed trauma). This observation shows the vital importance of an intact pain response. Affected children or carriers of Fabry disease can develop painful inherited sensory neuropathy associated with loss of almost all nociceptive nerve endings in the skin. Some painful small-fiber neuropathies of unknown cause (idiopathic) are present in multiple family members and likely have genetic causes that await investigation. Other acquired neuropathies are those associated with exposure to toxins such as heavy metals or to drugs, such as anticancer, antituberculosis, and antiretroviral agents.
etiology, distribution, and pathology. Some are specific to the type of axon involved, but many affect more than one type of axon (e.g., sensory-motor polyneuropathy). Motor symptoms typically include weakness and affect the distal muscles, often the extensor groups. Sensory disturbances can be classified by examination with pin, heat or cold, and vibratory stimuli. The distribution of neuropathies can be symmetrical or asymmetrical, multifocal or focal. In the polyneuropathies, neuronal dysfunction is first reported in the extremities, the distal portions of the longest axons. Often, the earliest symptoms are those of the autonomic and small-fiber sensory modalities. The diagnostic evaluation includes a thorough history to ascertain the etiology, including questions regarding systemic illnesses, injuries, nutritional deficiencies, family history, and toxin exposure. Lists of recommended diagnostic tests are available at http://www.neuroskinbiopsy.mgh.harvard.edu. Painful sensory neuropathies have been associated with systemic disorders such as human immunodeficiency virus (HIV) infection, diabetes mellitus, alcohol abuse, hepatitis, malignant cancers, monoclonal gammopathies (IgG, IgA, and IgM), rheumatoid arthritis, collagen vascular disease, and amyloidosis. Inherited neuropathies, such as the hereditary sensory and autonomic neuropathies (HSAN), can also cause neuropathic pain. HSAN-1 is the most common characterized HSAN seen in the United States. In severe neuropathies, there can also be complete absence of pain sensation with devastating consequences (mutilation secondary to repetitive and unnoticed trauma). This observation shows the vital importance of an intact pain response. Affected children or carriers of Fabry disease can develop painful inherited sensory neuropathy associated with loss of almost all nociceptive nerve endings in the skin. Some painful small-fiber neuropathies of unknown cause (idiopathic) are present in multiple family members and likely have genetic causes that await investigation. Other acquired neuropathies are those associated with exposure to toxins such as heavy metals or to drugs, such as anticancer, antituberculosis, and antiretroviral agents.
In the United States, the most common cause of painful neuropathies is glucose intolerance. It has become clear in recent years that neuropathy can be one of the earliest manifestations of diabetes and can even precede the development of serum abnormalities that are used to classify patients as having diabetes. Two-hour glucose tolerance testing has been shown to be a more sensitive method of detecting abnormalities of glucose metabolism in patients with neuropathy than tests of fasting plasma glucose levels or measurements of hemoglobin A1c. Diabetes causes several types of neuralgia, but those involving small fibers are most common. A patient with diabetic small-fiber neuropathy presents with “burning feet” and autonomic features including impaired thermoregulation and sweat production. Neuropathy and vascular insufficiency are the main risk factors for foot ulcers and amputation. In painful diabetic polyneuropathy, both myelinated and unmyelinated fibers can degenerate. Demyelination can be present as well. Endoneural vascular resistance, insufficient neurotrophic support, and autoimmune inflammation may all contribute to nerve damage. Diabetes mellitus also
causes other types of neuropathies, including proximal motor neuropathies due to intraneural vasculitis (diabetic amyotrophy), autonomic neuropathies, and vulnerability to compressive lesions; acute painful neuropathies from nerve ischemia, hypoglycemic neuropathy, treatment-induced neuropathy; and distal motor neuropathies. Patients with multifocal neuropathies (mononeuropathy multiplex) develop local loss of function in several peripheral nerves. Systemic lupus erythematosus, rheumatoid arthritis, cholesterol emboli, and polyarteritis nodosa, as well as diabetes mellitus, also can cause this pattern. The pathologic basis of these syndromes is nerve infarction from vasculitis. Prognosis for recovery is favorable if the underlying cause of infarction can be treated.
causes other types of neuropathies, including proximal motor neuropathies due to intraneural vasculitis (diabetic amyotrophy), autonomic neuropathies, and vulnerability to compressive lesions; acute painful neuropathies from nerve ischemia, hypoglycemic neuropathy, treatment-induced neuropathy; and distal motor neuropathies. Patients with multifocal neuropathies (mononeuropathy multiplex) develop local loss of function in several peripheral nerves. Systemic lupus erythematosus, rheumatoid arthritis, cholesterol emboli, and polyarteritis nodosa, as well as diabetes mellitus, also can cause this pattern. The pathologic basis of these syndromes is nerve infarction from vasculitis. Prognosis for recovery is favorable if the underlying cause of infarction can be treated.
(ii) Painful Mononeuropathies
Isolated focal peripheral nerve lesions are most often due to trauma. Although unintentional injuries are probably the most common cause, iatrogenic injuries from surgery or needle-stick are an underappreciated close second. Because a proportion of injuries occur on the job, or from participation in sports, these patients are likely to be young and in their most productive years, and are often male. Nerve injuries may not be diagnosed at the time of the initial accident because they are not visible on x-ray, and medical attention usually focuses on more obvious injuries. Clinicians should evaluate these patients with the aid of a handbook that demonstrates the individual nerve territories. Stewart’s textbook Focal Peripheral Neuropathies is an invaluable resource. It may be helpful to refer patients with difficult-to-diagnose syndromes to a neurologist or neurosurgeon with subspecialty training in peripheral nerve injury. Nerve lesions affecting predominantly sensory neurons and producing pain as the major symptom are less likely to be diagnosed than those that produce frank motor deficit as well. Delays in diagnosis can be unfortunate because patients with some types of peripheral nerve injuries benefit from early surgical nerve repair. Furthermore, failure to diagnose a nerve injury can result in repeated surgeries if the pain is erroneously attributed to other causes. Nerve injuries in patients without a history of trauma or surgery are usually the result of internal entrapment, compression, or nerve ischemia. Chronic nerve entrapment injury can be associated with rheumatic disease, diabetes mellitus, uremia, hypothyroidism, repetitive use, or malnutrition, or rarely occur in otherwise healthy individuals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







