Chapter 10
Neuropathic pain and complex regional pain syndrome
By the end of this chapter the reader should have an understanding of:
1 Pathophysiological mechanisms underlying neuropathic pain and complex regional pain syndrome.
2 Symptoms that alert the clinician to the possible presence of these conditions.
3 Current diagnostic criteria and their limitations.
4 A methodical approach to examination and treatment, based on current international evidence and guidelines.
Part 1 Neuropathic pain
INTRODUCTION
Neuropathic pain is defined by the International Association for the Study of Pain (IASP) as ‘pain caused by a lesion or disease of the somatosensory nervous system’ (International Association for the Study of Pain, 2011). The IASP no longer lists the term neurogenic pain, although in practice the two terms are often used interchangeably.
Neuropathic pain can have its origin in either the central or peripheral nervous system and can have a number of causes (Box 10.1). The diagnosis of neuropathic pain is not straightforward because it can mimic other painful conditions and present with seemingly contradictory signs and symptoms. This is further complicated by the fact that pain may have a nociceptive as well as a neurogenic component, requiring careful examination and possibly multimodal treatment.
Selecting the most efficient treatment can be even more difficult, as presentation and responses are extremely variable, even within one type of neuropathic pain. This has led to the suggestion that treatments should aim to address underlying mechanisms instead of aetiological categories (Woolf & Max 2001). A herculean effort by a German team has now established that instead patients can be grouped according to their responses to sensory testing, which is moving us closer to achieving this (Maier et al 2010).
MECHANISMS OF NEUROPATHIC PAIN
A few basic concepts are essential for the understanding of neuropathic pain. The first is the concept of projection: the intact sensory nervous system projects information to the cortex and other cerebral structures, where awareness arises. Signals coming in via nociceptive pathways are typically interpreted by the brain as pain stemming from a somatic problem. Damage anywhere along the sensory pathway alters nerve function and may lead to pain, which is erroneously interpreted by the brain as coming from the tissues at the peripheral end of the sensory line. The patient will therefore describe abnormal sensations in the body, and it is the task of the clinician to find out which ones are indeed coming from the body and which ones are generated from within the nervous system. For an overview of projection pathways, please refer to Chapter 5.
Any changes in the distribution or type of ion channel along the neuron, or degeneration of the myelin sheath, alters neuronal function and may cause neuropathic pain. For example, damage to peripheral nerves leads to dense concentrations of sodium channels along the axon (Devor et al 1994). This can produce ectopic impulse-generating sites that make the neurons depolarize spontaneously. Additionally it produces mechanosensitivity, which is demonstrated with Tinel’s test, for example for carpal or tarsal tunnel syndrome. This may be a risk particularly where nerves run close to tight or firm musculoskeletal structures (Devor 2006).
In relation to alterations in ion channel distribution, chemicals called neurotrophic factors are important. During prenatal development and after birth the tissues produce these neurotrophins, which draw the developing neurones towards them (Baccei & Fitzgerald 2006; Jessell & Sanes 2000). These factors either bind with tyrosine kinases (trk) receptors on the neural terminal, leading to the production of chemical messengers inside the neuron, or they are absorbed into the neural terminal. Messengers are constantly transported back to the cell body, where they affect protein synthesis, thereby determining the structures produced to make up the neuron, including the ion channels. Once the nervous system has stopped developing, the tissues’ neurotrophins continue to maintain the structure and function of the neurones that innervate them. Changes in this communication between tissue and neuron, for instance because of nerve compression, can alter the neuronal make-up and cause neurogenic pain.
Neurotrophic factors are also released by Schwann cells, macrophages and other cells as part of Wallerian degeneration, along with other inflammatory mediators (Devor 2006; Marchand et al 2005). This promotes sprouting of the proximal stump. As some sprouts connect with and grow into the original endoneuriums, the remaining ones die back. However, if growth is blocked (for instance by scar tissue) the sprouts may form a neuroma, which can be extremely sensitive. Sprouting has also been observed in adjacent uninjured neurones (collateral sprouting).
Axonal damage may lead to a loss of insulation of individual neurones within a nerve. Activity in an axon can now produce excitation of adjacent fibres, making it possible for pain to develop in the absence of local stimulation. This is known as crosstalk (Devor 2006). Additionally, coupling between sympathetic neurons and afferents has been described. This is the result of the formation of noradrenaline receptors along damaged afferent axons (Jänig & Baron 2002). It has the potential to increase a patient’s pain in response to sympathetic activity, for instance with mental and emotional stress, physical exertion or feeling unwell. If this mechanism is suspected, it is important to reassure the patient that their symptoms are not psychogenic.
Finally, central changes may contribute to neuropathic pain, as described in Chapter 6. Neuropathy can lead to the development of central sensitization, heightening the response to sensory stimulation and recruiting Aβ fibres into the generation on pain. Moreover, this is reinforced by reduced spinal and descending control (Dickenson & Bee 2008).
EXAMINATION
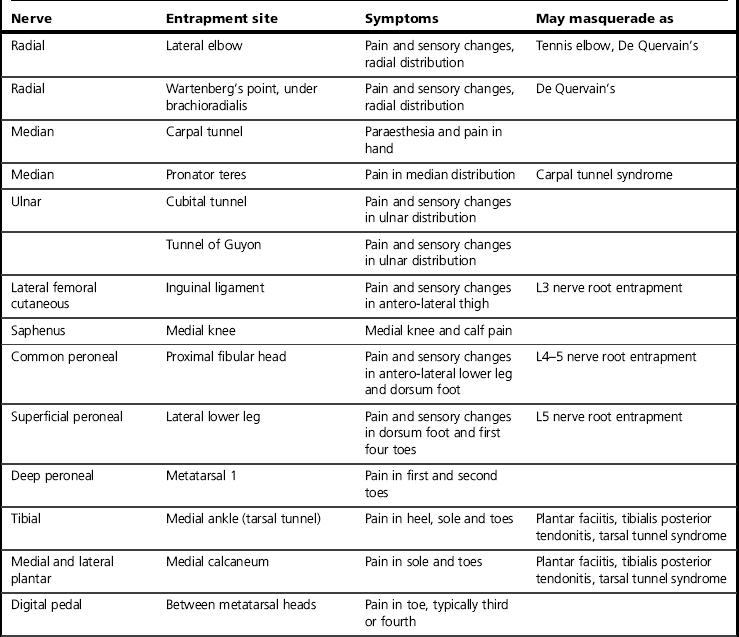
The clinical characteristics of neuropathy affect any or all components of a nerve and may therefore include sensory, autonomic or motor changes. Sensory changes may be wide ranging and include increased or decreased sensation or pain, allodynia, paraesthesia, dysaesthesia, spatial changes and temporal changes such as latency, aftersensation and summation (Hansson & Kinnman 1996). Neuropathies may present with different combinations of signs and symptoms, with large variations between individuals and conditions. Classic descriptions include burning and electrical pains as well as paraesthesia and numbness, but these symptoms are not always present, nor does their absence exclude neuropathy. Signs may well be paradoxical, such as intense pain felt in a cutaneous region that is numb to the touch. Finally, neuropathies may mimic, and occur alongside, other conditions (Table 10.1). It is for these reasons that careful subjective and objective clinical examination is essential. If there is any doubt about the diagnosis, a referral should be made to a neurologist or pain specialist (Dworkin et al 2007).
Careful history taking is the cornerstone of every examination. This may start with filling in a body chart, and it may be advantageous to ask the patient to do this. The exact signs and symptoms and the way they developed can suggest what the pathology and aetiology are. Concomitant diseases can also give an indication (see Box 10.1) and may require their own tests. Spontaneous as well as evoked signs and symptoms must be explored, as well as their exact localization on the body. Patients may be concerned that they may not believed because of the potentially contradictory nature of the symptoms, so it is essential to reassure them that what they report is not uncommon/understandable. Neuropathic symptoms and the way they behave are often strange to patients, so it is recommended that clinicians give patients a chance to formulate their own descriptions of symptoms without attempting to step in. At the end of history taking, the clinician should have at least one hypothesis regarding the underlying pathology.
A number of validated screening tools based on common features can aid the diagnosis (Table 10.2). The Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), Neuropathic Pain Questionnaire (NPQ), Douleur Neuropathique en 4 (DN4), painDETECT and ID-Pain all include questions about the most common neuropathic symptoms, such as tingling, electrical and burning sensations. The inclusion of other sensations or numbness varies by questionnaire. The LANSS and DN4 have the advantage of including a few basic sensory tests. The complexity of neuropathic presentation means that these tests can be used to assess the likely presence of neuropathy, but not to diagnose or evaluate treatment. A recent development is the use of the McGill questionnaire, which at present requires further evaluation (Dworkin et al 2009).
Table 10.2
Neuropathic screening tools (Bennett et al 2007)
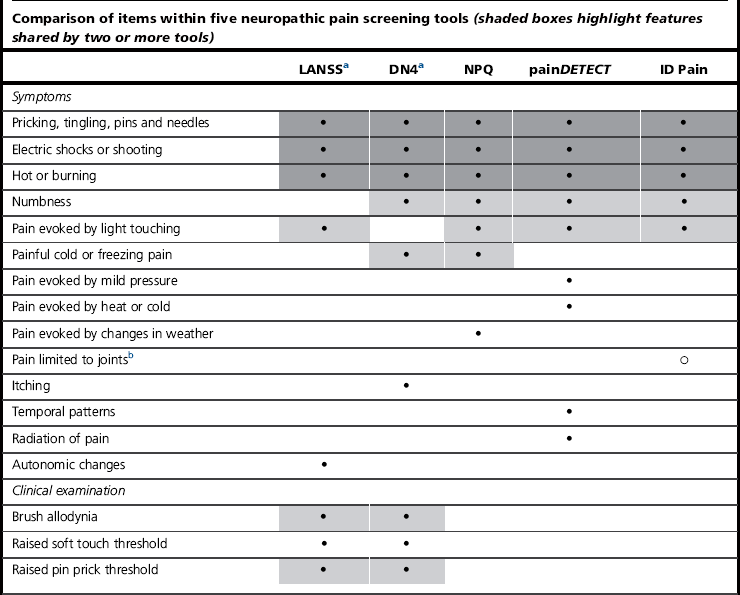
b Used to identify non-neuropathic pain.
aTools that involve clinical examination.
Bennett, M., Attal, N., Backonja, M., Baron, R., Bouhassira, D., Freynhagen, R., Scholz, J., Tölle, T., Wittchen, H., & Jensen, T., Using screening tools to identify neuropathic pain. PAIN, 2007, 127(3); 199-203.This table has been reproduced with permission of the International Association for the Study of Pain® (IASP). This table may not be reproduced for any other purpose without permission.
Sensory testing is the final part of the examination and aims to map out the exact distribution of sensory changes (for specific examples and potential difficulties see Hansson 1994). Ideally a number of tests are done because neuropathies do not always affect all sensory modalities, but basic tests include light touch, pinprick and proprioception. Sensory tests are briefly summarized in Box 10.2; for further reading refer to MacDermid (2005). In the case of positive features such as hyperaesthesia and allodynia, testing from outside the affected area towards its perimeter is recommended. However, negative features, i.e. reduced sensation, may be best tested from inside the zone going outwards.
Once a diagnosis of neuropathy has been established, a clear explanation of signs and symptoms to the patient is required. In some cases a treatment strategy can be discussed, although often the aim of further action is management and not a cure (British Pain Society 2008). If diagnosis or further management cannot be determined from the examination, specialized diagnostic techniques such as imaging, neurophysiological testing, quantitative sensory testing, thermography or diagnostic nerve block may be required (Hansson et al 2002, 2007).
TREATMENT AND MANAGEMENT OF NEUROPATHIC PAIN
There is little correspondence between disease or injury on one hand and specific mechanisms of neuropathy on the other. Efforts to identify which mechanisms play a role in each individual patient, and therefore which drugs have the best chance of success, are currently underway (Baron 2006b). Novel drug targets and other developments are reviewed in Dray (2008).
Treatment recommendations based on the available evidence are summarized in the section below; for a full review of mechanisms see Dworkin et al (2007) and Baron (2006b). Treatment objectives can be summarized as follows (Jensen 2008):
• Reduce peripheral sensitization.
• Decrease central sensitization.
• Reduce central facilitation and increase central inhibition.
Note that the latter has physiological as well as psychological components.
Overall approach
• A secondary amine tricyclic antidepressant TCA (nortriptyline, desipramine) or a selective serotonin and noreprinephrine reuptake inhibitor SSNRI (duloxetine, venlafaxine) (Kingery 1997). Explain to the patient that the treatment is not for depression.
• A ligand to voltage-gated calcium channel (gabapentin, pregabalin).
• For localized peripheral neuropathy, a lidocaine patch to target voltage-gated sodium channels (Black et al 2002; Galer et al 2002).
• For acute neuropathic pain, neuropathic cancer pain or severe episodic exacerbations, opioid medication or tramadol.
It must be noted that only partial pain relief can be expected in only half of the patients, and that side effects can form a significant prohibitive factor (O’Connor 2009). It is therefore important to also start non-pharmacological treatment as appropriate. There is low-level evidence for the use of transcutaneous electrical nerve stimulation (TENS; see Chapter 14) and spinal cord stimulation (SCS; Jensen 2008). Therapy intervention needs to be directed to address the key treatment objectives (Jensen 2008), with modalities chosen as appropriate to the patient. As with all forms of persistent pain, realistic understanding, coping strategies and family involvement are essential. Anxiety, depression and problems sleeping must be addressed as applicable.
Third, the clinician should reassess the pain and associated quality of life regularly and for a sufficiently long period of time – this varies per drug but is at least 3–4 weeks. Continuation of treatment is recommended if the pain drops to manageable levels and side effects are tolerable. If relief is less marked, a further component of the treatment options outlined above can be added (Rhodes 2011). On the other hand, if relief is only minor or non-existent try another form of treatment altogether.
Finally, if single and combined treatments fail, other forms of medication or referral to a specialist pain service must be considered. Second-line treatments are opioids and tramadol, while third-line include antiepileptics such as carbamazepine and lamotrigine, antidepressants like citalopram and paroxetine, mexilitine, N-methyl-D-aspartate (NMDA) receptor antagonists such as ketamine, and capsaicin cream. Unfortunately no treatments have been proved effective for lumbosacral radiculopathy, which is perhaps the most common form of neuropathic pain (Dworkin et al 2007).
Desensitization
This is a process of reducing hypersensitivity in an area by sensory bombardment of the nerve endings in order to decrease touch-evoked pain and discomfort (Waylett-Rendall 1995). For suggested mediums of treatment see Box 10.3.
Desensitization should be carried out for 5–10 minutes, a minimum of three or four times a day, but up to six times a day. To read further regarding desensitization, and for specific examples of desensitization protocols, see Waylett-Rendall (1995).
Sensory re-education
The patient can be given exercise tasks involving touch and recognition of textures, patterns and objects whilst using the assistance of visual feedback. This process uses activities where vision guides touch, in an attempt to reprogramme the brain after an insult to the nervous system. The earliest published sensory re-education programme was set out by Wynn-Parry in 1966 and used localization of touch and recognition of shapes and textures (Rosen et al 2003).
The basis of the exercises is to perform simple repetitive tasks with the aim of re-educating both the peripheral and central nervous systems (Box 10.4), relating the tasks to the function of the affected body part. In upper limb dexterity, stereognosis, manipulation etc. are especially important, while the lower limb requires tolerance to weight-bearing and a variety of different afferent inputs. See Wynn-Parry (1980, p. 226) for a more detailed description of training programmes for the upper limb.
All textures and particles used for desensitization can be used for sensory re-education, as well as small and large everyday objects and different surfaces. Using sensory re-education in isolation has shown disappointing results (Rosen et al 2003), so Lundborg and Rosen (2007) have proposed a two-phase approach. Phase 1 addresses cortical reorganization of the somatosensory cortex and aims to maintain the cortical hand representation. Phase 2 explores novel principles to enhance the effects of sensory training, using selective deafferentiation.
• observed movements, either of the contralateral side or another individual’s body part
• reading or listening to ‘action words’ relating to activities involving the body part
• mirror therapy (see Part 2 for more detail)
• use of a sensor glove (a glove that gives auditory feedback) or texture rods that are rubbed whilst being held close to the ear, such that the sound of touch can be heard.
Functional Magnetic Resonance Imaging (fMRI) has demonstrated that observing hands being touched activates visual as well as somatosensory areas in the brain cortex (Lundborg 2008). Activation of the somatosensory cortex by visual observation of touch has also been demonstrated in the lower extremity (Keysers et al 2004). Empirically, sensory re-education procedures can stimulate recovery of sensation and normalize sensory feedback in neuropathic pain that presents as altered sensations such as allodynia, hyperaesthesia and dysaesthesia amongst others.
Phase 2 involves the use of Eutectic Mixture of Local Anesthetics (EMLA) anaesthetic cream (liaise with the Multi-disciplinary team (MDT) if required) and uses the principle of brain plasticity to enhance traditional sensory re-education activities. The EMLA cream is applied to the area of the body adjacent to the deafferented area. This is thought to allow expansion of the adjacent cortical representation, increasing the effectiveness of sensory re-education to this area. In a double-blind, randomized clinical trial, the EMLA group showed a significantly improved tactile discrimination when compared with the control group (Rosen et al 2006).
Cortical remapping
Changes in the somatosensory cortex of the brain have been determined in response to both ongoing pain (Flor et al 1997) and injury to peripheral nerves (Jensen 2002; Lundborg & Rosen 2007; Rosen et al 2003). In order to address these cortical changes the principles used in Phase 1 of Lundborg & Rosen’s sensory re-education may be used (Lundborg & Rosen 2007). The graded motor imagery programme and mirror therapy can also be used (see Part 2).
Part 2 Complex regional pain syndrome
INTRODUCTION
The characteristics of CRPS were described in 1872 by S Weir Mitchell, and years of hypotheses and names for the condition followed (Box 10.5). CRPS often develops in response to minor trauma to the limbs (Box 10.6). The term CRPS was introduced in 1994 by the IASP in order to provide clarity of diagnosis and treatment (Merskey et al 1994; Stanton-Hicks et al 1995). It was chosen for the following reasons: