KEY POINTS
Neuromuscular disorders (NMDs) in critical care may be divided into those that precipitate admission to the ICU and those that arise during ICU management.
Many patients who present to the ICU as a result of an underlying neuromuscular disorder will have a previously defined diagnosis. However, when a patient presents with recent onset of acute or subacute bilateral muscle weakness, a broad differential diagnosis must be considered.
A rapidly progressive spinal cord lesion is the most important diagnosis to consider and immediately exclude in patients presenting with ascending or flaccid paralysis.
The maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), vital capacity (VC), and qualitative judgment of oropharyngeal function are the most important parameters to follow in patients with NMDs.
An effective cough is unlikely with a MEP <40 cm H2O and risk of hypercapnia increases when MIP is less negative than −30 cm H2O. A VC <30 mL/kg impairs secretion clearance and respiratory failure is common at values <15 to 20 mL/kg.
Sleep-related deterioration in alveolar ventilation resulting in hypercapnia and hypoxia is common in patients with respiratory muscle impairment.
Most patients with Guillain-Barré syndrome or myasthenia gravis of sufficient severity to precipitate ICU admission will benefit from treatment with plasma exchange or intravenous immunoglobulin.
Muscle biopsy is useful in the diagnosis of polymyositis, mitochondrial disease, and other myopathies, and should be considered when electrophysiologic and other testing does not offer a clear diagnosis of peripheral neuropathy or myoneural junction diseases.
NEUROMUSCULAR DISORDERS IN CRITICAL CARE: GENERAL ASSESSMENT AND MANAGEMENT
Neuromuscular weakness may result from disorders involving the peripheral nerves, neuromuscular transmission, or skeletal muscles. Neuromuscular disorders encountered in the critical care setting may be divided into those that result in ICU admission and those that are acquired during treatment of critical illness. Most patients who present to the ICU as a result of an underlying neuromuscular disorder will have a previously defined diagnosis. However, when a patient presents with recent onset of acute or subacute bilateral muscle weakness, a broad differential diagnosis must be considered (Table 87-1). The initial approach to differential diagnosis attempts to define the principal level of abnormality based on the patient’s history and findings on neurologic examination (Table 87-2). Additional diagnostic tests such as neuroimaging, nerve conduction, and electromyogram (EMG) studies are often needed to establish the underlying disorder more reliably. An easy to remember mnemonic, MUSCLES, may be helpful in remembering some of the most common causes of generalized weakness in the ICU1 (Table 87-3).
Causes of Acute and Subacute Bilateral Weakness
| Syndrome/Level of Abnormality | Representative Disorders |
|---|---|
| Basilar artery occlusion | Embolic, thrombotic, vasculitic |
| Myelopathy | Cord compression (eg, abscess, neoplasm, |
| disc herniation, trauma) | |
| Transverse myelitis | |
| Central nervous system infections | Poliomyelitis |
| West Nile virus | |
| Central nervous system toxins | Neurotoxic fish poisoning |
| Peripheral nerve disorders | Guillain-Barre syndrome |
| Phrenic nerve injury (eg, trauma, surgery neoplasm) | |
| Infections with phrenic nerve involvement (eg, diphtheria, herpes zoster, Lyme disease, West Nile | |
| Parsonage-Turner Syndrome with phrenic nerve involvement | |
| Heavy metal toxicity | |
| Vasculitic neuropathy | |
| Disorders of neuromuscular transmission | Myasthenia gravis |
| Eaton-Lambert syndrome | |
| Botulism | |
| Tick paralysis | |
| Organophosphate poisoning | |
| Penicillamine toxicity | |
| Myopathic disorders | Dermatomyositis/polymyositis |
| Metabolic myopathy (eg, mitochondrial disease) | |
| Toxic myopathy (eg, corticosteroid injury, alcohol, cocaine, rhabdomyolysis) | |
| Electrolyte disorders | Hypokalemia |
| Periodic paralysis | |
| Hypophosphatemia | |
| Hyperkalemia | |
| Hypermagnesemia | |
| Hypocalcemia |
Differential Diagnosis of Neuromuscular Disorders Leading to ICU Admission
| Level of Abnormality | Presentation | Representative Disorders | Nerve Conduction | EMG |
|---|---|---|---|---|
| Upper motor neuron |
|
| Normal | Normal |
| Lower motor neuron |
|
| Normal | Denervation |
| Peripheral nerve |
|
| Reduced | Denervation |
| Neuromuscular junction |
|
| Normal | Abnormal repetitive stimulation |
| Muscle |
|
| Normal | Small motor units |
Mnemonic for Differential Diagnosis of Generalized Weakness in the Intensive Care Unit
| M | Medications: steroids, neuromuscular blockers (cisatracurium, pancuronium, vecuronium), zidovudine, amiodarone |
| U | Undiagnosed neuromuscular disorder: myasthenia, LEMS, inflammatory myopathies, mitochondrial myopathy, acid maltase deficiency |
| S | Spinal cord disease (ischemia, compression, trauma, vasculitis, demyelination) |
| C | Critical illness myopathy, polyneuropathy |
| L | Loss of muscle mass (cachectic myopathy, rhabdomyolysis) |
| E | Electrolyte disorders (hypokalemia, hypophosphatemia, hypermagnesemia) |
| S | Systemic illness (porphyria, AIDS, vasculitis, paraneoplastic, toxic) |
Involvement of respiratory muscles is the most common reason that patients with primary neuromuscular disorders are admitted to the ICU. Although lesions involving the upper and lower motor neuron may occasionally be responsible, more often the underlying disorder affects the peripheral nerves (eg, Guillain-Barré syndrome, GBS), neuromuscular junction (eg, myasthenia gravis, MG), or skeletal muscles (eg, dermatomyositis and polymyositis, DM/PM). In this chapter, we will address neuromuscular disorders that may present with acute or subacute declines in respiratory muscle strength leading to acute respiratory failure. Our discussion will primarily focus on GBS, MG, and DM/PM. We will also offer a brief review of several additional disorders that should be considered in the differential diagnosis of patients presenting to the ICU with progressive neuromuscular impairment. Before discussing individual disorders, we will review the evaluation and management of respiratory muscle weakness.
There are three primary mechanisms by which respiratory failure develops as a direct consequence of an underlying neuromuscular disorder: (1) weakness of inspiratory muscle, particularly the diaphragm, (2) inadequate expiratory muscle function, and (3) impairment in muscles of the upper airway.2-4 The primary clinical consequences of impairment in one or more of these muscle groups include inadequate ventilation, nocturnal hypoventilation, ineffective cough, and aspiration of oropharyngeal secretions.
The diaphragm serves as the principal muscle involved in inspiration, but the external intercostal, sternocleidomastoid, scalene, and trapezii muscle groups also contribute. Inspiratory muscle weakness is most often gradual in onset, but can progress rapidly.2 Orthopnea is common due to the mechanical disadvantage placed on the diaphragm in the supine position, sometimes leading to an erroneous diagnosis of congestive heart failure. As diaphragm weakness progresses, use of accessory muscles of inspiration and paradoxical inward movement of the abdomen may be seen. Nocturnal hypoventilation is common with diaphragm weakness, particularly during rapid eye movement sleep when the accessory muscles of inspiration are inhibited. Eventually, alveolar hypoventilation results in hypercapnia and overt respiratory failure. Even though the main consequence of inspiratory muscle weakness is ineffective ventilation, weakness of the inspiratory muscles can also contribute to ineffective cough by limiting the degree of lung expansion and thereby the amount of pressure that can be generated by the expiratory muscles.
The dominant muscles used during active expiratory effort are the transversus abdominis, rectus abdominis, internal and external obliques, and the internal intercostals.2 Adequate expiratory muscle strength is essential for an effective cough and clearance of airway secretions. In addition, active expiration may aid inspiration when the diaphragm is weak by two mechanisms: forcing the diaphragm into a more favorable length-tension position and increasing the elastic recoil energy of the chest wall, both of which may enhance the forcefulness of subsequent inspiration.
Bulbar impairment greatly increases the risk for aspiration of oropharyngeal secretions, a common cause of acute respiratory failure in patients with progressive neuromuscular diseases. The coordinated action of muscles of the pharynx, palate, tongue, and larynx are required for normal swallowing and upper airway protection.2,5 In addition, weakness of the laryngeal muscles can contribute to ineffective coughing since incomplete glottic closure will prevent the generation of high intrathoracic pressure needed to expel mucus. Unfortunately, bulbar muscle impairment is often unrecognized, potentially resulting in increased morbidity and mortality.5 Assessment of oropharyngeal function is primarily based on clinical observation and early consultation with speech pathology is strongly recommended.
Early in the evolution of respiratory muscle weakness, patients may exhibit a paucity of symptoms, and objective testing is necessary. Maximal inspiratory pressure (MIP), maximum expiratory pressure (MEP), and vital capacity (VC) are the most important respiratory muscle parameters to follow.2,3,6,7 Using a combination of respiratory muscle tests offers greater diagnostic accuracy than relying on a single test result.8 Of note, slow VC is less altered by underlying airflow obstruction and is felt to be a better measurement of respiratory muscle weakness than forced VC. These tests should be followed frequently in hospitalized patients who have an evolving neuromuscular disorder, with careful attention to serial changes.9 Measurements of respiratory muscle strength are highly effort dependent. Appropriate procedural technique and adequate patient cooperation and effort are essential. The MIP and MEP are the most sensitive indicators of respiratory muscle strength. Measurement of MIP and MEP requires a maximal effort at residual volume (MIP) and total lung capacity (MEP), using a bedside manometer fitted with a mouthpiece. It is recommended that the MIP and MEP that is sustained for at least 1 second should be recorded rather than a transient spike in pressure. Normal values for MIP and MEP in adults aged 18 to 65 years are approximately −70 cm H2O and 100 cm H2O for women, and approximately −95 cm H2O and 140 cm H2O for men.6,10 Respiratory muscle weakness is suggested by MIP values less negative than −30 cm H2O for women and −45 cm H2O for men, and MEP values less than 60 cm H2O for women and 80 cm H2O for men.8 Normal predicted values for patients 65 years of age or older are reduced, and reference equations are available to define the lower limit of normal.11 The normal VC in adults is approximately 50 to 70 mL/kg.
Serial assessment of MIP, MEP, and VC are of greatest value in being able to identify patients who may require ventilator assistance before they experience an acute crisis with overt hypercapnic respiratory failure or even respiratory arrest. Threshold values have been primarily derived from observational studies of patients with GBS.9 Cough is likely to be ineffective when the MEP is <40 cm H2O, and there is risk of hypercapnia when the MIP is less negative than −30 cm H2O. Elimination of secretions with coughing is impaired when the VC declines to <30 mL/kg and a VC <15 to 20 mL/kg greatly increases the likelihood of respiratory failure.2,5,12
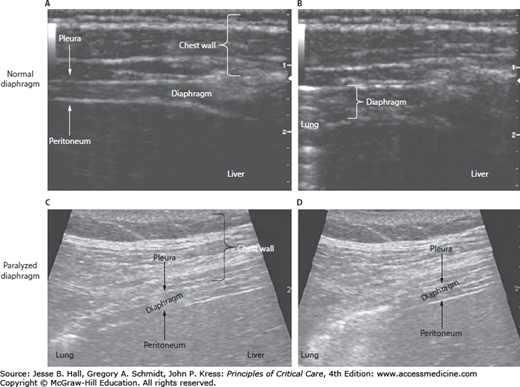
Recently, ultrasound of the diaphragm has been shown to be a useful noninvasive technique to assess for diaphragmatic paralysis.3,13,14 An ultrasound of the diaphragm at its zone of apposition with the rib cage normally reveals thickening of the diaphragm with inspiration secondary to diaphragmatic shortening during contraction. In the presence of diaphragmatic paralysis, the diaphragm does not shorten with inspiratory efforts (Fig. 87-1). In addition, a recent study defined the expected range of diaphragm excursion in normal individuals using ultrasound.14 Thus, diaphragmatic ultrasound may be used as both a diagnostic tool to assess for diaphragm paralysis and to monitor patients for recovery of diaphragmatic function.3,15
FIGURE 87-1
Ultrasound images of normal and paralyzed diaphragms. Panels A and B show the end-expiration and end-inspiration stages, respectively, in a normal diaphragm. Panels C and D show the end-expiration and end-inspiration stages, respectively, in a paralyzed diaphragm. During inspiration the normal diaphragm thickens, whereas the paralyzed diaphragm does not thicken. (Reproduced with permission from McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl Med. March 8, 2012;366(10):932-942.)
Sleep-related deterioration in alveolar ventilation, resulting in hypercapnia and hypoxemia, are common in patients with respiratory muscle impairment, particularly during rapid eye movement sleep.4,16,17 The reduction in VC normally seen in the supine position is greatly exaggerated in patients with respiratory muscle weakness. Normally, the VC declines by 5% to 10% in the supine compared with upright positions, and severe diaphragmatic weakness is suggested by a decline of ≥30%.7 When awake and upright, patients with respiratory muscle weakness may exhibit adequate gas exchange, but the latter may deteriorate significantly when they are sleeping supine. Indeed, sleep-related deterioration in gas exchange is often the earliest manifestation of respiratory muscle weakness in patients with neuromuscular disease.3,4 Furthermore, when patients have preexisting sleep disordered breathing, the development of neuromuscular disease may greatly exacerbate their underlying sleep disorder. When the supine VC is <60% of the predicted value, or the MIP less negative than −46 cm H2O (<4.5 kPa; 1 kPa = 10.19 cm H2O), sleep-disordered breathing is common.17
Ventilatory assistance is commonly required in patients with progressive respiratory impairment related to neuromuscular disease. In the absence of significant oropharyngeal dysfunction that dictates need for intubation for airway protection, noninvasive ventilation (NIV) may provide adequate ventilatory support.18 However, in one prospective study 37% of patients who received NIV for hypercapnic respiratory failure in the absence of obstructive airway disease eventually required intubation.19 In this study, refractory respiratory acidosis and depressed mental status were the most common reasons for intubation. In a study of patients with myasthenic crisis only hypercapnia with a [Math Processing Error] >45 mm Hg predicted BiPAP failure and subsequent intubation.20 Besides oropharyngeal dysfunction, altered mental status, moderate to large secretions with ineffective cough, inability to participate with application of NIV, and hemodynamic instability are indications for intubation and institution of mechanical ventilation in patients with progressive neuromuscular disease.
The use of sedative, analgesic, and neuromuscular blocking agents in patients with neuromuscular disease requires consideration of several important clinical and pharmacologic observations. Sedative and analgesic therapy may increase the risk of acute respiratory failure secondary to increased muscle weakness from the direct muscle relaxant effects of these agents, and depression of central respiratory drive.4 Ideally, patients with neuromuscular disease would be intubated and managed without the use of neuromuscular blockade. However, if neuromuscular blockade is necessary the following important pharmacologic principles need to be carefully considered. Succinylcholine should not be used to facilitate intubation of patients whose neuromuscular disease involves denervation.21 This would include patients with GBS, multiple sclerosis, amyotrophic lateral sclerosis, and those with a stroke or spinal cord injury more than 24 hours before intubation. In these patients, an upregulation of fetal-type acetylcholine receptors may result in life-threatening hyperkalemia after administration of succinylcholine. Patients with MG are commonly resistant to succinylcholine secondary to a reduction in the number of acetylcholine receptors or functional antibody-induced receptor blockade.21 In addition, secondary to significant reduction in functional receptors, patients with MG may be very sensitive to nondepolarizing agents.21 Although nondepolarizing neuromuscular blocking agents may be used in patients with GBS, an increase in sensitivity to these agents has been reported.21
Supportive care of critically ill patients with neuromuscular disease remains a central component of their management strategy in the ICU, including attention to deep venous thrombosis and stress ulcer prophylaxis, nutritional support, and skin care.22 In addition to speech therapy consultation, early consultation and close follow-up by physical and occupational therapists are also recommended. Patients should also be regularly assessed for pain, dyspnea, anxiety, and depression. Psychiatry consultation should be requested when appropriate. Daily communication with all members of the care team remains essential in optimizing clinical outcomes in this challenging patient population.
Weaning from mechanical ventilation should be considered only after clear evidence of improvement in general and respiratory muscle-specific weakness has been demonstrated. As muscle strength in patients with neuromuscular disorders may fluctuate, a durable improvement in respiratory muscle strength should be confirmed. Prospective studies are not available to clearly guide bedside decision making in patients with neuromuscular disorders, but improvement in VC to >20 mL/kg and MIP to more negative than −30 cm H2O are reasonable thresholds to achieve before considering extubation. Measurements of respiratory function (MIP, MEP, and VC) and ultrasound of the diaphragm may be helpful in assessing the patient who exhibits difficulty in weaning from mechanical ventilation.13 Adequate oropharyngeal function is an important element of successful extubation, and a thorough evaluation of oropharyngeal function should be performed following extubation and prior to resuming oral intake.
Tracheostomy will be necessary in patients whose neuromuscular disorder does not improve sufficiently to allow safe extubation.5,22 Timing of tracheostomy must be individualized, but is advisable when prolonged (>2-3 weeks) mechanical ventilation will be required. Studies involving patients with GBS and myasthenia gravis have identified certain risk factors for prolonged mechanical ventilation (see below).
Guillain-Barré Syndrome: Guillain-Barré syndrome is an acute inflammatory demyelinating polyneuropathy that most often presents with ascending symmetrical weakness beginning in the lower extremities.5,12,23,24 In approximately 10% of patients, weakness may be first noted in the upper extremities or facial muscles.12,25 Weakness typically evolves over days to weeks, although a subset of patients experience a rapid decline in function over hours. Excluding trauma, GBS is the most common cause for acute flaccid paralysis in previously healthy people.12 The ascending weakness is accompanied by depressed or absent reflexes. Sensory involvement is common, and the majority of patients experience peripheral paresthesias as their initial symptom. In addition, an aching discomfort in the lower back and legs may also be seen in the early phase of the syndrome.24,26 Autonomic dysfunction is common in patients with GBS, occurring in 70% of patients.5,22,24 Autonomic dysfunction may result in brady- or tachyarrhythmias, orthostatic hypotension, hypertension, or abnormal sweating. Life-threatening alterations in autonomic function, including arrhythmias and extreme hypertension or hypotension, develop in 20% of patients with GBS.12,22,24 Although bowel and bladder function are usually preserved, ileus and urinary retention can occur.
Variants of the typical GBS presentation may be encountered, including the Miller-Fischer variant, with ataxia, ophthalmoparesis, and areflexia.12,23,24 Overall, ophthalmoparesis develops in approximately 15% of patients. Acute inflammatory demyelinating polyradiculopathy (AIDP) is the most common presentation in the United States and Europe.24 However, variants of AIDP are encountered in 10% to 15% of patients. Primary axonal form of GBS, acute motor axonal neuropathy, and acute sensorimotor axonal neuropathy are more commonly seen in southeast Asia and Mexico.24
For unclear reasons, GBS appears to be more common in young adults and in the elderly. A preceding infectious syndrome with respiratory or gastrointestinal symptoms, usually occurring 1 to 4 weeks prior to the onset of neurologic symptoms, has been noted in approximately two-thirds of patients.12,23,25Campylobacter jejuni and cytomegalovirus infections are the most commonly identified triggers for GBS. In addition, Epstein-Barr virus, varicella virus, HIV, and Mycoplasma pneumoniae infections have also been associated with the development of GBS.24 A diverse and seemingly unrelated group of triggers have been identified, including infections, vaccination (eg, 1976 influenza vaccination), general surgery, epidural anesthesia, thrombolytic agents, drugs, neoplastic disease (Hodgkin disease), sarcoidosis, and connective tissue diseases.5,12,24 An autoimmune mechanism is strongly suspected in the pathogenesis of GBS; however, the immunopathology has not been fully defined.
A diagnosis of GBS is based on the clinical presentation and electrodiagnostic studies compatible with a demyelinating polyneuropathy.5,12,23,24 Elevated cerebrospinal fluid (CSF) protein levels are commonly noted after the first week of symptoms and is typically accompanied by a normal cell count or limited mononuclear pleocytosis (<10 cells/cm3). Pleocytosis in the CSF fluid appears to be more common in patients with human immunodeficiency virus infection and GBS.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree