Neurologic Complications of Peripheral Neural Blockade
Hervé Bouaziz
Dan Benhamou
The exponential increase in the utilization of peripheral nerve blocks (PNBs) began in the 1990s. This increase has been more marked (and earlier to occur) in Europe than in the United States (1). In France, a 1996 national survey showed that regional anesthesia was used in more than 20% of surgical procedures, a 14-fold increase in use when compared with data obtained in 1980 (2). Epidural and spinal anesthesia, which are associated with well-defined benefits in several patient populations, gained widespread acceptance during this interval. However, PNBs also were used more often, despite lack of definite and proven benefits. Conversely, the American Society of Anesthesiologists Closed Claims project confirmed the limited utilization of PNB in North America during this same period (3). For example, when claims between 1980 and 1999 were reviewed, PNBs accounted for 13% of all regional anesthesia claims and 21% of nonobstetric regional anesthesia claims. Axillary plexus blocks were used in the majority of cases followed by intravenous (IV) regional blocks and interscalene blocks (44%, 21%, and 19% respectively). Although the number of claims cannot be directly related to the frequency of use of each technique because denominators are not known, these data provide indirect evidence that a major evolution has occurred in the type of blocks used during the last decade.
With increasing utilization, case reports describing major morbidity or even death related to the use of PNB were reported. New technical developments, including stimulating peripheral catheters, ultrasound guidance, and portable infusion devices, were perceived to improve safety. However, safety is difficult to assess in the absence of large series of patients (4), and the real risk associated with these techniques remains unknown. Although most data are reassuring (5), others are less so (6), and the overall incidence of PNB-related severe morbidity and mortality remains unknown.
Incidence of Neurologic Complications and Identification of Etiology
Although PNBs have been practiced for years, large series of consecutive cases have only been gathered recently. This is in stark contrast to neuraxial blocks, for which several large series were published more than 50 years ago (7). As previously mentioned, this difference reflects the limited use of peripheral techniques compared to neuraxial blocks. However, with the increasing use of PNB during the last 15 years, investigators have started compiling total case numbers and their associated complications.
Incidence of Complications
Although the information provided by these series is invaluable, several limits should be acknowledged. Most series that have been reported are dominated by groups (or individuals) highly trained in regional anesthesia; intuitively, it may be suspected that complications are less frequently encountered in these practices. However, few data support this hypothesis. In a series of 1,000 consecutive axillary brachial plexus blocks (8) performed over a 12-year period, there was a 0.2% overall incidence of persistent paresthesia that fully recovered within 8 months. The occurrence of these complications did not decrease with increasing experience in contrast to the success rate, which increased significantly over the years. Other published reports come from university teaching hospitals and reflect the dynamics of learning, with subtle changes in the incidence of complications (and a likely reduction in the level of risk) over time. Numbers are often low, and conclusions drawn from rare complications should be treated with caution.
From the cases included in the closed-claims study (9), it is apparent that peripheral nerve injury may be associated with either regional or general anesthesia, since factors common to both—such as positioning, preexisting medical conditions, tourniquet use, and surgically induced complications—can be the cause of postoperative neurologic complications (10).
Etiology of Complications
Peripheral nerve techniques, even more than neuraxial blocks, are performed in the context of limb orthopedic surgery, which can itself produce complications. An example is hip replacement, which can lead to sciatic and/or femoral nerve lesion. When surgery is performed with the use of combined femoral and sciatic nerve block, controversy over which technique is at fault may arise when a postoperative neurologic injury occurs (11). Furthermore, neurophysiologic evaluation (electromyography, evoked potentials, nerve conduction studies) may not definitively differentiate the cause of the nerve trauma, as anesthetic and surgical injury are situated in the same site (12). By contrast, if sciatic nerve injury occurs after knee replacement
performed under combined sciatic–femoral nerve block, the site at which the nerve has been injured is often easier to recognize (site of needle insertion for regional anesthesia–related injury and level of the peroneal nerve for surgery-related injury). Separating the mechanisms of injury can also be made more difficult in orthopedic surgery because, during limb surgery, tourniquet use may lead to nerve/muscle trauma. In this context of difficult differential diagnosis to separate anesthetic and surgical technique as the cause of nerve injury, it is important to consider the respective incidence of lesions caused by each technique. For example, hip replacement (performed under general anesthesia) is associated with a 0.5% to 2% incidence of sciatic nerve lesion (13), and this high incidence has led several experts to use evoked potentials during surgery to facilitate early detection of nerve trauma (14). Sciatic and/or femoral nerve blocks are associated with nerve injury in one to two of 1,000 anesthetic procedures—an incidence much lower than that for surgery-induced nerve trauma. This comparison should lead one to consider that, in difficult-to-diagnose cases, a surgery-induced lesion should be considered first. A sciatic nerve lesion is more often encountered after revision/reoperation or acetabular reconstruction for dysplasia in females and with an inexperienced surgeon (13,15). When one or several of these risk factors exist, then a surgical etiology is more likely the cause of nerve injury than is the sciatic nerve block. By contrast, femoral nerve lesion can occur but has been very rarely described after hip replacement (16), leaving more uncertainty as to the cause of the injury.
performed under combined sciatic–femoral nerve block, the site at which the nerve has been injured is often easier to recognize (site of needle insertion for regional anesthesia–related injury and level of the peroneal nerve for surgery-related injury). Separating the mechanisms of injury can also be made more difficult in orthopedic surgery because, during limb surgery, tourniquet use may lead to nerve/muscle trauma. In this context of difficult differential diagnosis to separate anesthetic and surgical technique as the cause of nerve injury, it is important to consider the respective incidence of lesions caused by each technique. For example, hip replacement (performed under general anesthesia) is associated with a 0.5% to 2% incidence of sciatic nerve lesion (13), and this high incidence has led several experts to use evoked potentials during surgery to facilitate early detection of nerve trauma (14). Sciatic and/or femoral nerve blocks are associated with nerve injury in one to two of 1,000 anesthetic procedures—an incidence much lower than that for surgery-induced nerve trauma. This comparison should lead one to consider that, in difficult-to-diagnose cases, a surgery-induced lesion should be considered first. A sciatic nerve lesion is more often encountered after revision/reoperation or acetabular reconstruction for dysplasia in females and with an inexperienced surgeon (13,15). When one or several of these risk factors exist, then a surgical etiology is more likely the cause of nerve injury than is the sciatic nerve block. By contrast, femoral nerve lesion can occur but has been very rarely described after hip replacement (16), leaving more uncertainty as to the cause of the injury.
Similar risk ratios of surgery- and anesthesia-related nerve injury can be found in other situations. Lynch and co-workers observed an incidence of severe neurologic injuries of 4.3% after 417 total shoulder arthroplasty procedures (17). Of patients with a neurologic complication, only 17% had an interscalene block. In a study of 693 patients receiving an interscalene block, the incidence, distribution, and resolution of neurologic sequelae were determined using a standardized assessment that was performed repeatedly until the fourth postoperative week (18). In all but one case, symptoms were reported within the first 2 weeks and were only sensory. Neurologic symptoms were noticed in 4.2% of patients during the first month, but all had resolved within 4 to 6 weeks, except one case of brachial plexopathy that resolved in 12 weeks (1.4/1,000). A retrospective study of a total of 1,614 axillary blocks performed on 607 patients also reported that of the 62 nerve injuries, seven (11%) were related to the anesthetic technique whereas the remaining 55 (89%) were a result of the surgical procedure (19). These comparative data again show that surgically induced nerve injuries are generally much more common than those related to PNB.
Even minor surgery may lead to neurologic symptoms, as shown by a study that analyzed the postoperative course of 100 patients who had undergone ambulatory hand surgery after randomized use of either general anesthesia or axillary block using a transarterial technique (20). On postoperative day 1, 60% of patients of either group had paresthesia; the incidence declined to 12% at 1-year follow-up, still with no difference between the two groups. This reports also highlights the role surgery itself can play in obscuring the analysis of postoperative complications due to regional anesthesia.
Duration of Neurologic Dysfunction
An additional difficulty when counting PNB-induced neurologic complications relates to the fact that most lesions observed immediately after surgery resolve rapidly and do not lead to long-term sequelae. Sufficient patient numbers must thus be large enough to separate time-limited and long-lasting complications. Lesions are indeed most often caused by neurapraxia or axonotmesis than by neurotmesis, the prognosis for which is much more severe (12). Axonotmesis results from nerve disruption with endoneurium and other supportive tissue preserved. Complete recovery is expected more or less rapidly for these two lesions. Neurotmesis by contrast, reflects complete disruption of nerve and supporting connective tissue and its prognosis is poor. In contrast, neurapraxia results from a mild degree of injury with impulse conduction failure. Electromyography demonstrates an unaltered pattern combined with decreased conduction and increased latency. Assessment of neurologic complications therefore requires calculating both early and late incidences of events. In a prospective survey, 521 patients scheduled for elective shoulder surgery performed with an interscalene block (6) were assessed at regular intervals, with the final evaluation at 9 months. Although 14% reported some neurologic abnormality unrelated to surgery on the tenth day, only one patient (0.2%) had symptoms remaining at 9 months. The high incidence of early symptoms (i.e., 14%) is only an approximation, as neurologic symptoms evolved as a dynamic condition. Spontaneous resolution was rapid in most patients, leaving an incidence of only 7.9% at 1 month. By contrast, in 0.2% of patients, symptoms became apparent only 2 to 3 weeks after the procedure, depending on the formation of perineural edema, inflammation, and microhematoma (6).
The extreme situation (i.e., high initial incidence of neurologic symptoms and extremely rare late complications) is found with the risk of diaphragm dysfunction after interscalene block. Diaphragmatic paresis is present in 100% of patients in the first hours after the block (21), explaining why this technique is contraindicated in patients with limited respiratory reserve. It was traditionally thought that no long-term sequelae exist. There are, however, a few recent reports describing permanent diaphragmatic paralysis after an apparently uneventful interscalene block (22,23).
In 1997, the first prospective large series of regional anesthetic procedures was reported (24). In this series, 21,278 PNBs were performed by experienced anesthesiologists over a 6-month period. All peripheral blockade–related neurologic complications were present on the second postoperative day. With an incidence of 1.9 per 10,000 cases, peripheral techniques were less likely associated with a neurologic complication than spinal anesthesia (5.9/1,000) and were complicated by neurologic injury at a rate similar to that of epidural anesthesia (2/10,000, NS). The vast majority of these neurologic symptoms had recovered at 3 months. More recently, the same group (25) reiterated their analysis of complications observed after regional anesthesia and surveyed prospectively 50,223 peripheral blocks also performed by highly trained anesthesiologists. Although only 12 patients had a peripheral neuropathy after a peripheral block (2.3/10,000), seven of them had sequelae still present after 6 months. Only serious adverse events were recorded, explaining why the incidences reported were much lower than in smaller series, which reported even mild symptoms (6,18). Patients had their neurologic injury diagnosed through the usual follow-up (usually surgeon-based), and it remains possible that some complications were not reported to anesthesiologists, suggesting that underestimation might exist. This concern may however be unjustified, as shown by a prospective survey of 5,147 patients who had undergone surgery using PNB for anesthesia (26). Patients were evaluated at 24 hours and again at 7 days after surgery. An overall incidence of four per 10,000 neurologic complications was found, consistent with incidences found in previous reports.
Table 20-1 Comparative incidence of neurologic complications after continuous peripheral nerve block (CPNB) in published studies | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Continuous Peripheral Nerve Blocks
In the large series by Auroy and colleagues (25), patients were included in 1998–1999, a time period during which the use of continuous PNBs was still limited. However, several series have evaluated the incidence of neurologic complications following the use of continuous peripheral techniques (Table 20-1). In an important study, 1,416 patients were followed in the postanesthesia care unit and every day for up to 5 days, to examine efficacy and complications related to the use of continuous catheters (27). Twelve patients (0.84%) experienced serious adverse events and three (0.21%) patients had neurologic lesions attributed to the continuous peripheral nerve catheter. All three nerve injuries were seen after a femoral nerve block, perhaps reflecting the fact that this technique was used in half of the surveyed patients. Nerve damage resolved 36 hours to 10 weeks later. A similarly relatively high incidence can be extracted from the report by Bergman and colleagues, who reported a series of 405 axillary catheters (28), two of which were associated with new and anesthesia-related neurologic injury (0.5%). Although the absolute number in these two studies is low, the incidence may be ten times greater than after single-injection nerve blocks. These results are refuted by Borgeat and co-workers (6), who reported the incidence of neurologic complications was 30% to 50% less after continuous (compared to single-injection) interscalene block. In both groups, however, a similar pattern of progressive decrease in the number of patients with persistent neurologic symptoms was observed. The same authors noted a similar complication rate in another prospective study that included 700 interscalene catheters (29).
Complications Associated with Selection of Peripheral Technique
It has been suggested that, even more so than the placement of a catheter, the insertion site for interscalene block might be a risk factor for an increased incidence and/or prolonged duration of complications compared to other techniques. For example, no late neurologic complications occurred in a prospective study involving 500 popliteal blocks (263 single-injection and 237 continuous techniques) (30). Likewise, in a large series of 3,396 peripheral blocks, the incidence of postoperative neurologic dysfunction was the lowest after axillary plexus blocks and the largest after interscalene block (4.0%, 2.0%, and 1.0%: interscalene, femoral–sciatic, axillary, respectively) although statistical differences were not significant regarding interscalene block (31). By contrast, Auroy and co-workers (25) found a greater incidence of neurologic complications following popliteal blocks than after interscalene blocks, whereas Borgeat and colleagues, prospectively studying 1,001 consecutive popliteal blocks, did not record any anomaly at 10 days and again at 3 months’ follow-up (32). These data suggest that needle insertion site (specific block) is difficult to associate with a high or low incidence of neurologic complications.
Mechanisms of Neurologic Injury
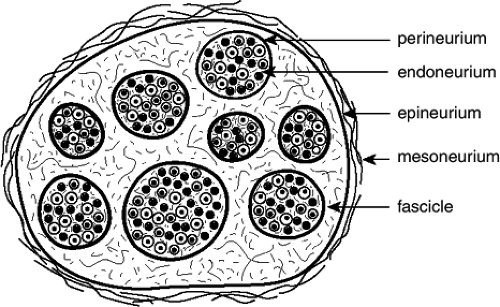
Neural damage is a possible complication of central and PNB and may significantly impact the quality of life of patients. Injury may be due to ischemic, mechanical, or chemical mechanisms—all of these factors occurring alone or in combination—and may aggravate preexisting nerve lesions (26,33). Trauma may disrupt neural blood vessels or neural barriers, cause extra- or intraneural hematoma or edema, and lead to degenerative changes or discontinuity of fibers. Intrafascicular injection (within the perineurium) (Fig. 20-1) is more likely to be associated with injury than intraneural extraperineural injection (34). Unduly high concentrations or intrafascicular injections of local anesthetics may lead to severe nerve injury. Histopathologic studies have shown that all local
anesthetics induce a concentration-dependent breakdown of the blood–nerve barrier, with concomitant edema and degenerative changes of nerve fibers (35). By contrast, extrafascicular injection results in a low risk, if any, of nerve damage (36) (Table 20-2).
anesthetics induce a concentration-dependent breakdown of the blood–nerve barrier, with concomitant edema and degenerative changes of nerve fibers (35). By contrast, extrafascicular injection results in a low risk, if any, of nerve damage (36) (Table 20-2).
Table 20-2 Intra- or extrafascicular injection as a risk factor of nerve injury | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Preexisting Patient Factors
Patients with preexisting peripheral nerve disorders may be at risk of neurologic injury after receiving a PNB, according to the “double-crush” theory described first by Upton and McComas (37). As postulated by these authors, the presence of a more proximal lesion renders the more distal nerve trunk more vulnerable to compression, with subsequent damage that far exceeds the expected additive injury caused by each isolated insult. Therefore, the performance of PNBs in patients with preexisting peripheral nerve disease may theoretically place them at increased risk of occurrence of a double-crush syndrome. In streptozotocin-diabetic rats, local anesthetic requirement is reduced and the risk of local anesthetic–induced nerve injury is increased during sciatic block (38). Moreover patients with diabetes may be theoretically at greater risk of nerve ischemia after receiving epinephrine due to the underlying microangiopathy. Although, it is not possible to conclude whether or not these patients are at greater risk of developing new or worsening neurologic symptoms after PNB, clinicians should consider lower concentrations of local anesthetic and assess the risks–benefit ratio before adding epinephrine to the anesthetic solution in these patients. In a large retrospective study, a higher success rate after a popliteal sciatic nerve block has been observed in diabetic patients (n = 371) versus nondiabetic patients (n = 971) (39), suggesting a higher sensitivity of nerve fibers to local anesthetic in diabetic patients. No difference was observed, however, regarding neurologic complications (there were none in either group), in contrast with animal studies discussed earlier (38). Another retrospective study compared patients with preexisting neuropathy who underwent ulnar nerve transposition under regional anesthesia (n = 100) or general anesthesia (n = 260) and also found a similar incidence (6%) of new or worsening postoperative neuropathy (40). More recently, analysis of a large cohort of 5,147 patients showed that diabetes mellitus and preexisting peripheral neuropathy were independent risk factors for the occurrence of paresthesia/dysesthesia at 24 hours after a PNB, as well as for prolonged duration of motor and sensory block (26). Furthermore, nerve stimulation does not always provide easy electrolocalization in patients with preexisting nerve disease, even at high current intensity (41,42). It thus seems reasonable to consider the individual risk–benefit ratio when deciding which anesthetic technique should be preferred in a patient with preexisting peripheral nerve disease.
Direct Trauma from the Injection Needle/Catheter
Elicitation of paresthesia may be used intentionally for PNBs and even when other methods are used, unintentional paresthesia may occur in as often as 40% of cases (43). The classic controversy regarding relative safety of elicitation of a paresthesia compared to other methods (mainly nerve stimulation) remains undetermined. Auroy and colleagues reported that all cases of persistent paresthesia after PNBs occurred in the same territory as the associated paresthesia (25). Lee and co-workers, in evaluation of the ASA Closed Claims database, recorded 13 neuropathies associated with a peripheral block (3); a paresthesia occurred before injection in four of these cases and during injection in two additional cases. Candido and colleagues also disclosed that paresthesia at needle insertion was an independent predictor of postoperative interscalene block–related sequelae (18). By contrast, no regional anesthetic technique risk factors, including elicitation of a paresthesia, selection of local anesthetic, or addition of epinephrine, were identified by Horlocker and co-workers; however, similar to Auroy and colleagues (25), the nerve deficit occurred in the distribution of an elicited paresthesia in five of the seven patients (19).
Multiple stimulation techniques (including withdrawal and redirection of the stimulating needle) as well as performance of repeated regional blocks (even within 1 week), which both theoretically may increase the rate of neurologic injury, do not seem to be risk factors for the occurrence of nerve injury (19,31). The deleterious role of paresthesia elicitation is indeed difficult to ascertain even in large series in which paresthesia
elicitation is used in every patient, as neurologic complications only occur in a very small percentage of cases.
elicitation is used in every patient, as neurologic complications only occur in a very small percentage of cases.
Interpreting the Role of Paresthesia in Nerve Injury
Elicitation of a paresthesia has been considered to be the surrogate marker of contact between the needle and the nerve. Unfortunately, recent studies demonstrate that the problem is more complex. First, definition of paresthesia is variable among authors, and patients often find it difficult to explain precisely the sensation felt. Second, a recent ultrasound study demonstrated that paresthesia is reported in only 37% of patients, and limb movement is obtained in 78% of patients at the time of direct needle-to-nerve contact (44), suggesting that nerve contact does not necessarily trigger paresthesia. As well, in studies in which paresthesia is the clinical end-point used to perform a brachial plexus block, nerve stimulation does not always produce muscle movement when paresthesia is obtained (45). Paresthesia may also be frequently associated with intraneural injection (observed using ultrasound guidance) but with no postoperative nerve injury, most likely because of extra-perineural injection (46). Likewise, during nerve stimulation at low current intensity (0.2–0.4 mA), patients do not spontaneously report paresthesia, although half of them describe electrical paresthesia on careful questioning (47). Klein and co-workers have recently provided an additional possible explanation as to why elicitation of a paresthesia is not always associated with motor response, and they describe the mechanism of nerve injury that can occur while using a nerve stimulation technique (48). Using a mathematical model of nerve stimulation, it was calculated that an intense stimulation close to the nerve (paresthesia suggesting close proximity) can block propagation. This could explain clinical situations in which the needle is advanced quickly at relatively high current and fails to elicit motor response until gradually withdrawn. In addition, nerve injury might then occur during needle movement with no motor response.
Techniques of Neural Localization
Direct prospective comparisons have also been unable to solve the controversy. In a nonrandomized study, Selander and co-workers enrolled a total of 533 patients—290 in the paresthesia group and 243 in the transarterial group (43). Clinical evidence of nerve damage occurred in 2.8% of the patients in the paresthesia group and in 0.8% in the artery group, the difference being not statistically significant. More recently, a study compared the incidence of complications using electrical stimulation or mechanical paresthesia for nerve localization in 218 patients who underwent interscalene block for shoulder surgery (49). The risk of developing postoperative neurologic symptoms was again comparable between the two methods. It should be stressed, however, that in these clinical studies, statistical power may have been too low to ensure that true differences become significant because of the low rate of complications with either technique, thus maintaining the uncertainty. Nevertheless, it is well documented that the use of a nerve stimulator does not guarantee that such complications will not occur and that nerve injury can occur with either method, even in the hands of experienced anesthesiologists.
Needle Bevel and Design
Bevel design and needle size may also affect the risk of nerve injury. Indeed, it has been found both in vivo and in vitro that a nerve fascicle tends to roll or slide away from an advancing needle point, especially when the needle is short-beveled (45 versus 14 degrees) (50). On the other hand, should a nerve fascicle become impaled, the lesions induced by a short-beveled needle (27 degrees) are more severe and take longer to repair than those induced by a long-beveled needle (12 degrees); introduction of the needle bevel transverse (compared to parallel) to the nerve fibers further increases the resultant injury (51). Others recommend the use of a tapered injection needle after having shown that this design produced the least damage after nerve puncture (52). Nevertheless, the relatively long distance between the tip of the needle and the side orifice results in local anesthetic solution injection at a site distant from the nerve. To solve this problem, a long-tapered double needle has been developed and compared to the beveled needle (Quincke), short-tapered needle (Whitacre), and long-tapered needle (Sprotte) with encouraging results. However, the ability of different bevels to penetrate the nerve or to push them away in the clinical setting is still not known. It should be noted that, in particular situations, the nerve can be potentially pinned between needle tip and bony structure, such as during block of the ulnar nerve at the elbow and common peroneal nerve at the neck of the fibula (53). Finally, the short-bevel needle may be preferred as it is easier to discern the crossing of fascia, thus enabling the delivery of local anesthetics in the right place.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree