Neurologic
3-1 Intracranial Hemorrhage
Allon Amitai
Clinical Presentation
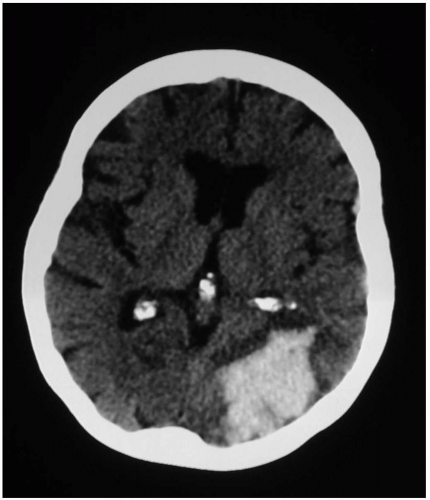
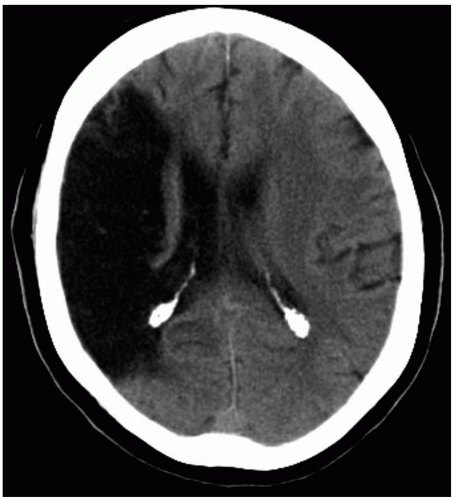
Patients with intracranial hemorrhage (ICH) typically present with headache, nausea, and vomiting, with or without focal neurologic deficits. Onset of ICH is gradual in comparison to ischemic infarct or subarachnoid hemorrhage (SAH). Altered level of consciousness may arise from increased intracranial pressure (ICP), direct damage to the reticular activating system, or herniation.1
Pathophysiology
Most ICHs occur spontaneously, secondary to long-standing hypertension.1 Age, hematoma volume, presenting Glasgow Coma Scale (GCS) score, and presence of intraventricular blood are the most important prognostic variables.2 Common locations for ICH are the basal ganglia, external capsule, cerebellum, and brainstem secondary to rupture of perforating arteries. However, hemorrhages in the posterior fossa carry a substantially worse prognosis than supratentorial bleeds and often require surgical decompression.1 Identified risk factors include anticoagulation, thrombolysis, antiplatelet therapy, African-American race, end-stage renal disease, hypocholesterolemia, vasculitis, amyloidosis, diabetes mellitus, and abuse of alcohol, tobacco, heroin, or sympathomimetics.1 Structural disorders such as arteriovenous malformation, intracranial aneurysm, and neoplasia are frequently encountered in younger patients. ICH may also develop from hemorrhagic conversion of ischemic stroke or from trauma.
Diagnosis
Noncontrast computed tomography (CT) detects most clinically significant acute ICHs and differentiates ICH from ischemic infarct. CT can also indicate the need for interventions such as ventriculostomy placement or surgical decompression.1
Clinical Complications
Complications include herniation, obstructive hydrocephalus, seizures, disequilibrium of centrally regulated homeostasis, syndrome of inappropriate secretion of antidiuretic hormone (SIADH), and aspiration. Between 30% and 50% of patients presenting with ICH die within 1 month, and, by 6 months, only 20% will be functioning independently.1
Management
Patients who are unable to protect their airway and those with rapidly progressing neurologic deficits should be intubated. Blood pressure should be reduced to a mean arterial pressure (MAP) of 130 mm Hg to maintain cerebral perfusion while reducing the risk of extension of bleeding. Hyperglycemia should be treated. Antiseizure prophylaxis is indicated only if there is clinical evidence of seizure. Midline shift and mass effect may be partly alleviated by administration of mannitol, diuretics, and corticosteroids; elevation of the head of the bed; and hyperventilation to a carbon dioxide tension (PCO2) of 35 mm Hg. Angiography or magnetic resonance imaging (MRI) is indicated in younger patients who are likely to have surgically treatable structural pathology. Urgent neurosurgical consultation and admission to an intensive care unit (ICU) are usually indicated.1,2
REFERENCES
1. Panagos PD, Jauch EC, Broderick JP. Intracerebral hemorrhage. Emerg Med Clin North Am 2002;20:631-655.
2. Dubinsky I, Penello D. Can specific patient variables be used to predict outcome of intracranial hemorrhage? Am J Emerg Med 2002;20:26-29.
3-2 Subarachnoid Hemorrhage
Cherie Mininger
Clinical Presentation
Patients with nontraumatic subarachnoid hemorrhage (SAH) present with the “worst headache of their life,” the so-called thunder-clap headache with sudden onset, usually during exertion.1,2 Patients may exhibit a depressed level of consciousness, transient loss of consciousness, nausea, vomiting, nuchal rigidity, and focal neurologic signs.1,2 They may also provide history of prior severe headache, known as a warning or “sentinel” headache.2
Diagnosis
Diagnosis is made by computed tomography (CT) in 93% of patients, if the scan is done within 24 hours after headache onset.2 Patients with a normal CT scan and a high index of suspicion for SAH should have a lumbar puncture performed. The cerebrospinal fluid (CSF) should be evaluated for both erythrocytes and xanthochromia. There is no set number of red blood cells needed in the CSF to exclude SAH.1 Xanthochromia is considered to be the most important CSF finding supporting the diagnosis.2
Pathophysiology
Clinical Complications
In some instances, the initial presentation is catastrophic. In other cases, especially with delays in diagnosis, patients may have a course complicated by rebleeding (4%), hydrocephalus, seizures, or vasospasm.2,3 Rebleeding is associated with a 70% mortality rate and is the most concerning immediate complication.3
Management
REFERENCES
1. Edlow JA. Diagnosis of subarachnoid hemorrhage in the emergency department. Emerg Med Clin North Am 2003;21:73-87.
2. Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med 2000;342:29-36.
3. Fahy BG, Sivaraman V. Current concepts in neurocritical care. Anesthesiol Clin North Am 2002;20:441-462.
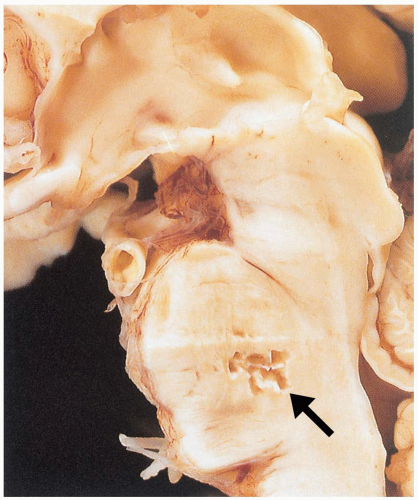
3-3 Cerebellar Hemorrhage
Cherie Mininger
Clinical Presentation
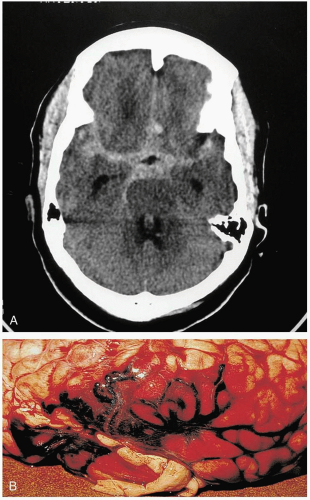
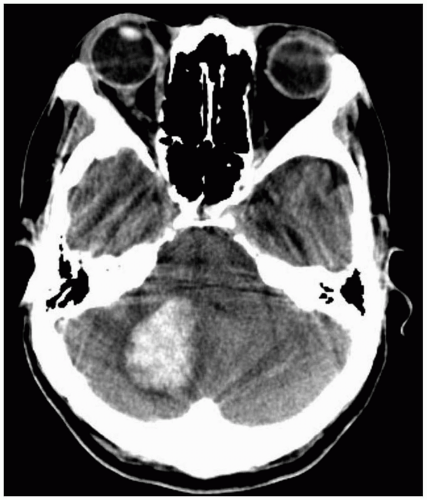
Patients with cerebellar hemorrhage (CBH) present with nonspecific complaints of headache, vomiting, and neck stiffness.1,2,3 Initial findings may include nonspecific findings of altered consciousness and elevated blood pressure.1 Findings specific to CBH include ataxia, nystagmus, and dysmetria.1,3
Diagnosis
Diagnosis is made by computed tomography (CT). If there is concern for CBH, the CT scan must include thin cuts through the posterior fossa, so that small cerebellar hemorrhages are not missed.2
Pathophysiology
Clinical Complications
Management
Patients with CBH require aggressive airway management, because most have a depressed mental status.3 Urgent neurosurgical consultation should be obtained, because these patients may be candidates for emergency surgical decompression.1,2,3 Intensive care unit (ICU) admission is warranted for management of the airway, blood pressure, and ICP, as well as seizure prevention.1
REFERENCES
1. Panagos PD, Jauch EC, Broderick JP. Intracerebral hemorrhage. Emerg Med Clin North Am 2002;20:631-655.
2. Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin 2000;18:419-438.
3. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450-1460.
3-4 Cerebrovascular Accident
Rachel Haroz
Clinical Presentation
Patients experiencing a cerebrovascular accident (CVA) may present with abrupt onset of a variety of neurologic problems, including vision loss, headache, and change in mental status. Patients may also develop respiratory compromise and hemodynamic instability. Transient ischemic attacks (TIAs) are focal neurologic deficits that resolve within 24 hours. There are about 750,000 new CVAs annually in the United States, with more than 150,000 fatalities, making CVA the third leading cause of death and the leading cause of disability.1 Risk factors include hypertension, heart disease, diabetes, high cholesterol, male gender, age older than 55 years, family history, smoking, and pregnancy. African-American race is also a risk factor.1,2
Pathophysiology
A CVA occurs when cerebral blood flow is acutely disrupted to a focal area, leading to decreased supply of oxygen and glucose. The underlying cause may be ischemic (85%), caused by a thrombotic or embolic occlusion, or hemorrhagic (15%), creating a cascade of events that leads to decreased energy production, acidosis, and, ultimately, cell death.2
Diagnosis
The diagnosis is made based on examination and history. Electrocardiography (ECG) and echocardiography are helpful to rule out atrial fibrillation, thrombus, and myocardial infarction. A computed tomography (CT) or magnetic resonance imaging (MRI) scan of the head should be performed. The differential diagnosis includes seizures, brain tumors, migraines, hypoglycemia, and hypertensive encephalopathy.1
Clinical Complications
Potential complications include seizures, sepsis, pneumonia, deep vein thrombosis, respiratory depression, pulmonary embolus, decubitus ulcers, and urinary tract infections.1
Management
The cause of the CVA (hemorrhagic or ischemic) determines the treatment. Patients with significant symptoms and ischemic strokes, less than 3 hours of symptoms, and no contraindications may be candidates for thrombolytic therapy. Other treatments include heparin, aspirin, and IIB/IIIA inhibitors. Hemorrhagic strokes should be treated supportively, and consultation with a neurosurgery service should be obtained.1,2
REFERENCES
1. Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med 2000;343:710-722.
2. Lewandowski C, Barsan W. Treatment of acute ischemic stroke. Ann Emerg Med 2001;37:202-216.
3-5 Lacunar Cerebral Vascular Accident
Erica McKernan
Clinical Presentation
Lacunar cerebral vascular accidents (CVAs) (infarcts) are small (less than 15 mm in diameter), deep infarcts that result from occlusion of a penetrating artery. These subcortical infarcts are located primarily in the basal ganglia, thalamus, internal capsule, corona radiata, and brainstem.1,2,3
If a lacunar infarct is symptomatic, the patient will present complaining of a motor and/or sensory deficit. Five well-described clinical lacunar syndromes are associated with lacunar infarcts: pure motor hemiparesis, sensorimotor stroke, pure sensory stroke, dysarthria or clumsy hand syndrome, and ataxic hemiparesis. Involvement of the face, arm, and leg are characteristic of the pure motor hemiparesis, sensorimotor stroke, and pure sensory stroke syndromes.1,2,3
Pathophysiology
Lacunar infarcts account for about 25% of strokes. These infarcts are small, deep, intraparenchymal infarcts that result from occlusion of penetrating arteries. Occlusion of the vessel may be caused by intracranial atherosclerosis or lipohyalinosis secondary to chronic hypertension. Lacunar infarcts are typically located in the basal ganglia and thalamus. Risk factors include age, gender, hypertension, diabetes mellitus, smoking, previous TIA, and possibly ischemic heart disease.2
Diagnosis
Most lacunar infarcts are clinically silent. In patients with neurologic deficits, neuroimaging can aid in the diagnosis of lacunar stroke. Because these infarcts are small, they usually are not seen on computed tomography (CT). Magnetic resonance imaging (MRI) is the study of choice.
Clinical Complications
The risk of death immediately after stroke onset is low, and the rate of recovery is usually rapid. Lacunar strokes have the best short- and long-term prognoses, with 30-day mortality rate of 2%, and the lowest risk for early recurrence of stroke. Late recurrence of stroke is about 14% at 3 years.3 Patients have an increased risk of developing cognitive decline and dementia.2
Management
Airway, breathing, and circulation must be ensured and assessment of neurologic function completed. Neurology consultation should occur immediately for further management.1
REFERENCES
1. Thurman RJ, Jauch EC. Acute ischemic stroke: emergent evaluation and management. Emerg Med Clin North Am 2002;20:609-630.
2. Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol 2003;2:238-245.
3. Sacco RL, Wolf PA, Gorelick PB. Risk factors and their management for stroke prevention: outlook for 1999 and beyond. Neurology 1999;53[7 Suppl 4]:S15-S24.
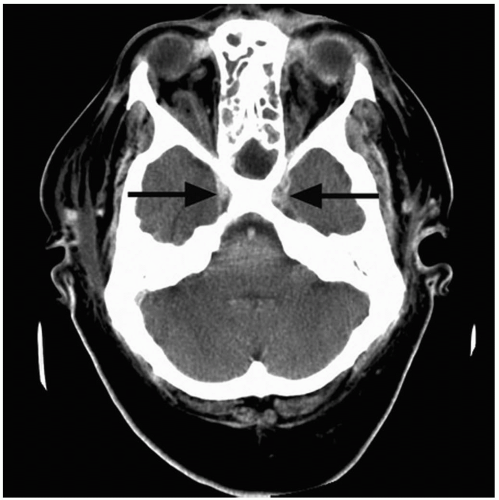
3-6 Cavernous Sinus Thrombosis
Lekha Shah
Clinical Presentation
Patients with cavernous sinus thrombosis (CST) may appear quite ill with abrupt onset of fever, headache, nuchal rigidity, nausea, vomiting, and eye pain.1,2 Testing of the cranial nerves may reveal unilateral or bilateral palsies of nerves III, IV, and VI, as well as sensory deficits in the ophthalmic branch of the trigeminal nerve. Eye findings may include orbital edema and tenderness, retinal hemorrhages, papilledema, proptosis, and dilated or sluggish pupils.1,2 Visual deficits may progress to blindness.1,2
Pathophysiology
CST is usually caused by a blood-borne infection extending from the face, ear, nasal cavity, or paranasal sinuses.3 Seeding often occurs via the superior and inferior ophthalmic veins, which drain the middle portion of the face.1,2 The end result is thrombosis and inflammation of structures within or surrounding the cavernous sinus: the internal carotid artery, cranial nerves III and IV, and the V1 and V2 branches of the trigeminal nerve.1,2 Staphylococcus aureus is the most commonly isolated organism, followed by Streptococcus species.1,2
Diagnosis
Clinical Complications
Management
REFERENCES
1. Volturo GA, Repeta RJ Jr. Non-lower extremity deep vein thrombosis. Emerg Med Clin North Am 2001;19:877-893.
2. Goldberg AN, Oroszlan G, Anderson TD. Complications of frontal sinusitis and their management. Otolaryngol Clin North Am 2001;34:211-225.
3. Ebrigth JR, Pace MT, Niazi AF. Septic thrombosis of the cavernous sinuses. Arch Intern Med 2001;161:2671-2676.
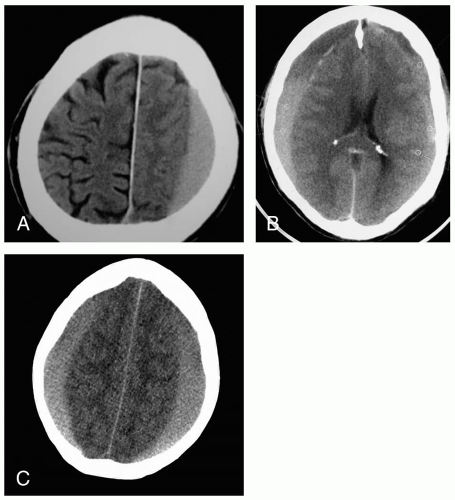
3-7 Acute Subdural Hematoma
Treve Henwood
John Queen
Clinical Presentation
Acute subdural hematoma (ASDH) occurs in approximately 30% of severe head injuries and results in altered consciousness in almost all cases. Headache, vomiting, or lethargy (97% to 99%); hemiparesis (34% to 47%); and papillary irregularity ipsilateral to hematoma (47% to 53%) may also occur. Patients may have a lucid interval before neurologic symptoms appear (30%).1
Pathophysiology
Bleeding results from tearing of bridging veins between the cerebral cortex and the draining venous sinus, resulting in increased intracranial pressure, narrowing of ventricles due to clot volume, and edema secondary to brain injury.2
Diagnosis
The history focuses on the mechanism of injury (direct versus acceleration/deceleration) and the neurologic states before and after injury. Radiographic findings from noncontrast head computed tomography (CT) include a crescent-shaped, high-density blood collection between the brain and dura. Loss of sulci and narrowing of the ventricles may be seen, and a midline shift occurs secondary to large clot volume.2,3
Clinical Complications
Secondary brain injury occurs after the initial trauma as a result of damage to neurons and is the leading cause of morbidity and mortality. Elevated ICP, brain edema, recurrent bleeding, and seizures are potential complications; they affect 33% of patients treated in the emergency department (ED).3
Management
Minimizing secondary brain injury and obtaining early neurosurgical intervention (within 4 hours) optimize outcomes. Early intervention in patients with a Glasgow Coma Scale (GCS) score lower than 8 should include rapid-sequence intubation. Short-term hyperventilation is recommended only for patients who are rapidly deteriorating. The target mean arterial pressure should be greater than 90 mm Hg. Hypertonic saline in the initial resuscitation of head-injured patients has shown some short-term benefit in lowering the ICP, but it is not currently recommended for all patients.2,3,4 Patients who are triaged or transferred early to trauma centers have the best overall outcomes.2,3,4
REFERENCES
1. Klun B, Fettich M. Factors influencing the outcome in acute subdural haematoma: a review of 330 cases. Acta Neurochir (Wien) 1984;71:171-178.
2. Marik PE, Varon J, Trask T. Management of head trauma. Chest 2002;122:699-711.
3. Zink BJ. Traumatic brain injury outcome: concepts for emergency care. Ann Emerg Med 2001;37:318-332.
4. Servadei F. Prognostic factors in severely head injured adult patients with acute subdural haematomas. Acta Neurochir (Wien) 1997;139:279-285.
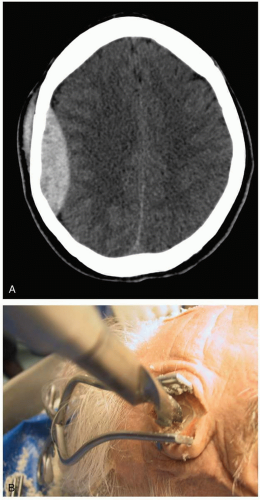
3-8 Epidural Hematoma
Cherie Mininger
Clinical Presentation
Patients with epidural hematoma (EH) experience a brief loss of consciousness after head trauma, followed by a lucid interval and subsequent neurologic deterioration.1,2
Diagnosis
The diagnosis is made radiographically; computed tomography (CT) shows a lenticular lesion, often in the temporal or temporoparietal region.1
Pathophysiology
EH is not common; it occurs in fewer than 1% of all head trauma cases.1 The injury is caused by laceration of the middle meningeal artery, middle meningeal vein, or dural sinus,2 with or without associated skull fracture. Bleeding from an EH can cause compression, shift, and increased intracranial pressure (ICP).2
Clinical Complications
Complications may be primary or secondary. Primary complications, resulting from direct mechanical injury, cause axonal injury manifested by an initial loss of consciousness or depressed mental status.1,2 Secondary complications, resulting from the expanding hematoma, cause neurologic deterioration.2
Management
Patients with EH require emergency neurosurgical evaluation and evacuation of the hematoma. The priority in managing the head-injured patient focuses on limiting secondary complications. Airway, breathing, circulation, and cervical spine stabilization must be addressed immediately. Any patient with a Glasgow Coma Scale (GCS) score of 8 or less, and any patient who is unable to protect his or her airway, should be intubated early, with the use of rapid-sequence techniques to limit fluctuations in ICP.2
REFERENCES
1. Marik PE, Varon J, Trask T. Management of head trauma. Chest 2002;122:699-711.
2. Gedeit R. Head injury. Pediatr Rev 2001;22:118-124.
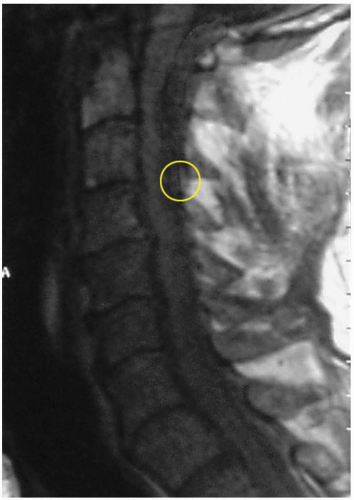
3-9 Spinal Epidural Hematomas
Matthew Warner
Clinical Presentation
Patients with a spinal epidural hematoma (SEH) present with the acute onset of local pain at the site of the hematoma. Neurologic symptoms are dependent on the location of the expanding mass.1 Progression from radicular paresthesia to paraplegia or quadraplegia, loss of sensory function, and death can occur.2
Pathophysiology
SEH is a rare condition, with only 260 reported cases.3 SEH may be secondary to trauma or an underlying medical condition (hypertension, coagulation disorders, anticoagulation medications, vascular malformations, collagen vascular disease), or it may be spontaneous, without any underlying cause. In all cases, bleeding originates from the venous plexus of the epidural space. Bleeding may also arise from hemorrhage of the epidural arteries. Bleeding from arteries is more likely to produce cord compression. Most SEHs are in the posterolateral position, the location of the epidural arteries. Traumatic SEHs are most often in the cervical region, whereas spontaneous SEHs are mostly in the lumbar region or distal to the spinal cord.1,2
Diagnosis
Magnetic resonance imaging (MRI) appears to be superior to computed tomography (CT) in the diagnosis of SEH, because MRI more clearly displays the hematoma and the extent of spread.2,3 The differential diagnosis includes spinal abscess, tumor, ischemia, transverse myelitis, and acute vertebral disk disease.2
Clinical Complications
Management
The definitive treatment of spinal epidural hematoma is laminectomy with surgical decompression.2 Permanent impairment depends on the time interval between onset of symptoms and surgery; only patients who undergo decompression within 12 hours after onset are expected to show significant improvement in neurologic function. However, there are case reports of patients with SEH managed medically. These patients all had minor neurologic deficits that were nonprogressive.3
REFERENCES
1. Marinella MA, Barsan WG. Spontaneously resolving cervical epidural hematoma presenting with hemiparesis. Ann Emerg Med 1996;27:514-517.
2. Alexiadou-Rudolf C, Ernestus RI, Nanassis K, Lanfermann H, Klug N. Acute nontraumatic spinal epidural hematomas: an important differential in spinal emergencies. Spine 1998;23:1810-1813.
3. Lefranc F, David P, Brotchi J, De Witte O. Traumatic epidural hematoma of the cervical spine: magnetic resonance imaging diagnosis and spontaneous resolution. Case report. Neurosurgery 1999;44:408-410.
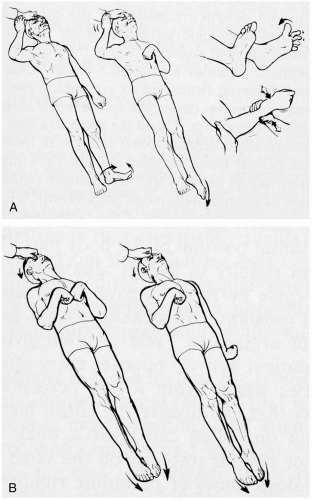
3-10 Decorticate and Decerebrate Posturing
Arthur Chang
Clinical Presentation
In decorticate posturing, patients display rigidity, with the arms flexed at the elbow and held inward and close to the body; the wrists and fingers are clenched and held tightly toward the chest; the legs and feet are usually extended. In decerebrate posturing, patients are rigid, with extension and internal rotation of the arms and legs, and plantar flexion of the feet. These signs can be intermittent, can be unilateral or bilateral, or can involve the upper extremities only. They may be seen in patients with severe traumatic vascular injury to the central nervous system (CNS) and in patients with hypoglycemia, cerebral edema, or diffuse axonal injury.1,2
Pathophysiology
Decorticate and decerebrate posturing result from damage to the structures that control motor tone associated with the corticospinal tract. In decorticate posturing, the lesion is rostral to the midbrain (i.e., cortex); in decerebrate posturing, the damage is at the level of the midbrain or caudal diencephalons.1 A progression from a decorticate state to a decerebrate state is associated with progressive destruction or compression of brain structures.1,2
Diagnosis
Emergency noncontrast computed tomography (CT) of the head should be done after initial stabilization of the patient. Magnetic resonance imaging (MRI) may better delineate cerebral edema or tumor progression, but it should be reserved until the patient is stable.1,2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree