Anatomy
 Endocrine physiology
Endocrine physiology
 Pituitary pathology
Pituitary pathology
 Pathophysiology of mass effect
Pathophysiology of mass effect
 Pathophysiology of endocrine hypersecretion
Pathophysiology of endocrine hypersecretion
 Therapy
Therapy
 Perioperative management
Perioperative management
 Complications
Complications
I. ANATOMY
The pituitary gland is housed within the sella turcica, a boney alcove of the sphenoid. Critical structures surrounding the pituitary gland and sella turcica (Fig. 12.1) include
A. Optic nerve and chiasm, which lie superior to the diaphragma sella
B. Cavernous sinus, which surrounds the sella turcica laterally
1. Internal carotid artery
2. Occulomotor (III) Nerve
3. Trochlear (IV) Nerve
4. Ophthalmic division of the Trigeminal (V1) Nerve
5. Maxillary division of the Trigeminal (V2) Nerve
6. Abducens (VI) Nerve
II. ENDOCRINE PHYSIOLOGY
A. Hypothalamus
1. The hypothalamus serves two primary physiologic roles within the body:
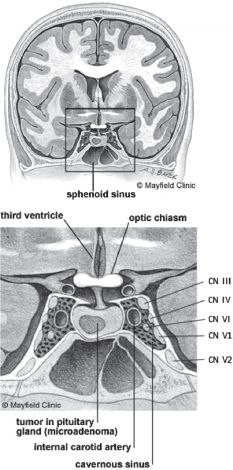
FIGURE 12.1 Anatomy of pituitary gland, cavernous and sphenoid sinuses. CN, cranial nerve. Copyright permission pending.
a. Regulation of the anterior pituitary gland through the synthesis of releasing and inhibiting factors
b. Production of oxytocin and vasopressin
2. Releasing and inhibiting factors, produced in response to feedback from target organs, travel to the pituitary gland via the hypothalamohypophyseal portal system.
3. At the level of the pituitary gland, releasing factors stimulate the synthesis and the secretion of hormones into the systemic circulation. Inhibiting factors modulate further hormone production.
B. Anterior pituitary gland (adenohypophysis)
1. The anterior pituitary gland produces hormones in response to a variety of intrinsic and extrinsic stimuli. The body’s diurnal rhythms and emotional wellbeing affect hormone production as do anesthetic drugs, surgical stress, and the hypothalamus.
2. Adenomas involving the anterior pituitary gland may lead to excessive production and secretion of one or more hormones. Those most commonly affected are adrenocorticotrophic hormone (ACTH), growth hormone (GH), and prolactin.
3. Radioimmunoassays detect and measure all anterior pituitary hormones in the systemic circulation.
4. Anterior pituitary gland hormones (Table 12.1)
TABLE 12.1 Anterior Pituitary Gland Hormones
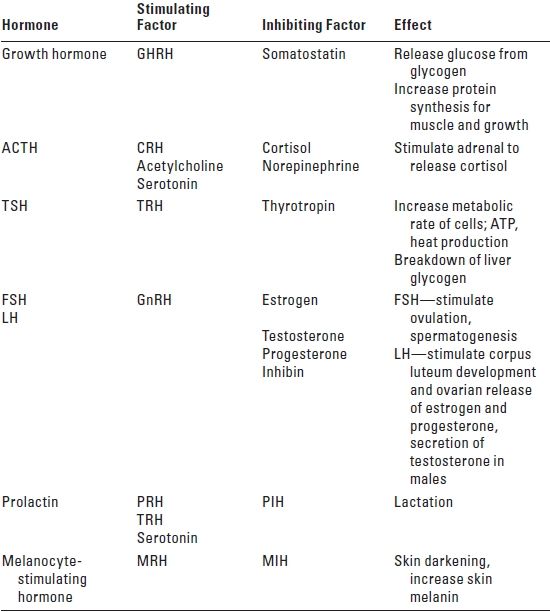
GHRH, growth hormone-releasing hormone; ACTH, adrenocorticotrophic hormone; TSH, thyroid-stimulating hormone; TRH, thyroid-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; GnRH, gonadotrophin-releasing hormone; PRH, prolactin-releasing hormone; PIH, prolactin-inhibiting hormone; MRH, melanocyte-releasing hormone; MIH, melanocyte-inhibiting hormone; CRH, cortisol-releasing hormone.
a. ACTH
b. Follicle-stimulating hormone (FSH)
c. GH
d. Luteinizing hormone (LH)
e. Prolactin (PRL)
f. Thyroid-stimulating hormone (TSH)
C. Posterior pituitary gland
1. Posterior pituitary gland hormones
a. Antidiuretic hormone (ADH) also known as vasopressin
b. Oxytocin
2. Both ADH and oxytocin are produced in the supraoptic and the paraventricular nuclei of the hypothalamus. Oxytocin differs from ADH in two of the nine amino acids.
3. Transport to the posterior pituitary gland occurs by way of axonal flow through the median eminence and pituitary stalk. The posterior pituitary gland serves as a reservoir for ADH and oxytocin. Both hormones are packaged into granules and secreted along with carrier proteins called neurophysins.
4. Numerous physiologic and pharmacologic factors influence ADH synthesis and secretion although hypovolemia has the greatest effect. (See Section IV for discussion of diabetes insipidus [DI])
III. PITUITARY ADENOMA: EPIDEMIOLOGY AND CLASSIFICATION
A. Epidemiology
1. The incidence of pituitary adenomas is difficult to determine. Clinically, they represent up to 10% of neoplasms affecting the brain. However, 27% of autopsies reveal a previously undiagnosed pituitary adenoma.
2. Forty percent of diagnosed pituitary adenomas are prolactinomas. Thirty-five percent are endocrine non-functioning pituitary adenomas.
3. Presentation and diagnosis typically occur between 30 and 50 years of age. Ten percent of diagnosed adenomas involve adolescents.
B. Classification
1. Size
a. Microadenomas measure less than 10 mm in diameter.
b. Neoplasms that are greater than 10 mm at the time of diagnosis are considered macroadenomas.
2. Location
a. Microadenomas generally remain within the confines of the sella turcica and are considered extracranial or extra-arachnoid.
b. Macroadenomas often disrespect the normal boundaries of the pituitary gland, extending into the intracranial space to reach the cavernous sinus, sphenoid sinus, and suprasellar area.
3. Functional versus non-functional
a. Functional adenomas often result from the neoplastic growth of a single cell type. Excessive production of one pituitary hormone occurs and accounts for presentation and diagnosis.
b. Macroadenomas are generally larger at the time of diagnosis. Such non-functional adenomas present secondary to signs and symptoms of mass effect.
c. Differential diagnosis
1. Adenoma
2. Craniopharyngioma
3. Meningioma
4. Carotid aneurysm
5. Optic glioma
6. Empty sella syndrome
7. Neoplasms
a. Dermoid
b. Germinoma
c. Metastatic lesion
8. Inflammatory disease
a. Infection
b. Sarcoid
c. Hemochromatosis
d. Amyloid
IV. PITUITARY ADENOMA: PATHOPHYSIOLOGY OF MASS EFFECT
Pituitary adenomas that extend beyond the normal boundaries of the sella turcica often produce symptoms secondary to mass effect, which causes the compression of surrounding structures. Signs and symptoms include:
A. Headache
Patients frequently complain of bitemporal or bifrontal headaches secondary to
1. Dural stretch of the diaphragma sella
2. Disruption of normal cerebrospinal fluid (CSF) flow through the third ventricle (with resultant hydrocephalus)
B. Visual field defects
1. Occur secondary to compression of the optic chiasm
2. Bitemporal hemianopsia is the classic presentation but decreased visual acuity is not uncommon
C. Cranial nerve palsy
1. May occur secondary to encroachment on the cavernous sinus by an enlarging mass
2. Double vision due to effect on Cranial Nerves III, IV, and VI
3. Facial numbness due to effect on Cranial Nerve V (V1 and V2)
D. Panhypopituitarism/hypothalamic dysfunction
1. Compression of normal pituitary tissue and/or disruption of the normal communication pathways between the hypothalamus and the pituitary gland may produce signs and symptoms of panhypopituitarism or DI.
2. Panhypopituitarism may include hypothyroidism, hypoadrenalism, and/or hypogonadism.
E. Diabetes insipidus
1. DI occurs when either mass effect or neoplastic infiltration compromises function of the supraoptic nuclei of the hypothalamus, resulting in diminished or absent ADH release. Under normal conditions, osmoreceptors in the supraoptic nuclei and stretch receptors in the left atrium respond, respectively, to an increase in osmolality or a decrease in the volume of the extracellular fluid compartment with the synthesis and the release of ADH. In the systemic circulation, ADH binds to specific receptors in the collecting tubules of the kidney, modulating water permeability.
2. Absent or diminished ADH produces:
a. Polydipsia
b. Hyperosmolality (> 320 mOsm/kg)
c. Hypernatremia (serum Na > 150 mEq/L)
d. Polyuria (> 3 L/day)
e. Dilute urine (urine specific gravity < 1.005 and osmolality < 200 mOsm/L)
3. Diagnosis relies upon recognition of the above signs and symptoms. Confounding conditions include diuresis secondary to hyperglycemia, osmotic diuretics such as mannitol, and overhydration.
4. Management of DI intraoperatively includes:
a. Determination of serum and urine electrolytes
b. Replacement of total body water deficit
c. Administration of aqueous vasopressin (see Section V below)
F. Hydrocephalus
G. Pituitary apoplexy
1. Pituitary apoplexy describes a condition of marked and often rapid expansion of a pituitary neoplasm and/or normal gland tissue secondary to hemorrhage, infarction, or necrosis.
2. Signs and symptoms of pituitary apoplexy include retro-orbital headache, ophthalmoplegia, visual deficits including blindness or decreased acuity, altered mental status from hydrocephalus, meningismus, and panhypopituitarism.
3. Addisonian crisis may occur in the face of acute ACTH deficiency leading to stupor, cardiovascular collapse, hyponatremia, and hyperkalemia.
4. Acute and symptomatic pituitary apoplexy with evidence of either hydrocephalus or visual defects requires surgical decompression. Adjunctive therapy includes corticosteroids.
V. PITUITARY ADENOMA: PATHOPHYSIOLOGY OF ENDOCRINE HYPERSECRETION
A. Cushing’s disease: ACTH hypersecretion
1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






